当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative Cytotoxicity of Inorganic Arsenite and Methylarsenite in Human Brain Cells.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-02-12 , DOI: 10.1021/acschemneuro.9b00653 Kunie Yoshinaga-Sakurai , Ravikumar Shinde , Myosotys Rodriguez , Barry P. Rosen , Nazira El-Hage
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-02-12 , DOI: 10.1021/acschemneuro.9b00653 Kunie Yoshinaga-Sakurai , Ravikumar Shinde , Myosotys Rodriguez , Barry P. Rosen , Nazira El-Hage
|
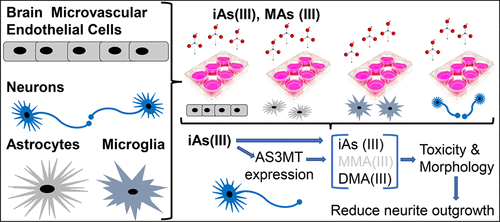
The overall goal of this study is to elucidate the potential effect(s) of arsenic on a variety of human brain cells. Arsenic is the most pervasive Group A human environmental carcinogen. Long-term exposure to arsenic is associated with human diseases including cancer, cardiovascular disease, and diabetes. More immediate are the health effects on neurological development and associated disorders in infants and children exposed to arsenic in utero. Arsenic is metabolized in various organs and tissues into more toxic methylated species, including methylarsenite (MAs(III)), so the question arises whether the methylate species are responsible for the neurological effects of arsenic. Arsenic enters the brain through the blood-brain barrier and produces toxicity in the brain microvascular endothelial cells, glia (astrocytes and microglia), and neurons. In this study, we first assessed the toxicity in different types of brain cells exposed to either inorganic trivalent As(III) or MAs(III) using both morphological and cytotoxicity cell-based analysis. Second, we determined the methylation of arsenicals and the expression levels of the methylation enzyme, As(III) S-adenosylmethionine (SAM) methyltransferase (AS3MT), in several types of brain cells. We showed that the toxicity to neurons of MAs(III) was significantly higher than that of As(III). Interestingly, the differences in cytotoxicity between cell types was not due to expression of AS3MT, as this was expressed in neurons and glia but not in endothelial cells. These results support our hypothesis that MAs(III) is the likely physiological neurotoxin rather than inorganic arsenic species.
中文翻译:

无机亚砷酸盐和甲基亚砷酸盐在人脑细胞中的比较细胞毒性。
这项研究的总体目标是阐明砷对多种人脑细胞的潜在作用。砷是最普遍的人类A类环境致癌物。长期接触砷与人类疾病有关,包括癌症,心血管疾病和糖尿病。对子宫内砷暴露的婴幼儿神经发育和相关疾病的健康影响更为直接。砷在各种器官和组织中代谢为毒性更大的甲基化物质,包括甲基亚砷酸盐(MAs(III)),因此出现了一个问题,即甲基化物质是否对砷的神经系统作用负责。砷通过血脑屏障进入大脑,并在大脑微血管内皮细胞,神经胶质细胞(星形胶质细胞和小神经胶质细胞)和神经元中产生毒性。在这项研究中,我们首先使用基于形态学和细胞毒性的分析方法评估了暴露于无机三价As(III)或MAs(III)的不同类型脑细胞的毒性。其次,我们确定了几种类型的脑细胞中砷的甲基化以及甲基化酶As(III)S-腺苷甲硫氨酸(SAM)甲基转移酶(AS3MT)的表达水平。我们表明,MAs(III)对神经元的毒性显着高于As(III)。有趣的是,细胞类型之间的细胞毒性差异不是由于AS3MT的表达,因为它在神经元和神经胶质中表达,但在内皮细胞中不表达。这些结果支持了我们的假设,即MAs(III)是可能的生理神经毒素,而不是无机砷。我们首先使用基于形态学和细胞毒性的分析方法评估了暴露于无机三价As(III)或MAs(III)的不同类型脑细胞的毒性。其次,我们确定了几种类型的脑细胞中砷的甲基化以及甲基化酶As(III)S-腺苷甲硫氨酸(SAM)甲基转移酶(AS3MT)的表达水平。我们表明,MAs(III)对神经元的毒性显着高于As(III)。有趣的是,细胞类型之间的细胞毒性差异不是由于AS3MT的表达,因为它在神经元和神经胶质中表达,但在内皮细胞中不表达。这些结果支持了我们的假设,即MAs(III)是可能的生理神经毒素,而不是无机砷。我们首先使用基于形态学和细胞毒性的分析方法评估了暴露于无机三价As(III)或MAs(III)的不同类型脑细胞的毒性。其次,我们确定了几种类型的脑细胞中砷的甲基化以及甲基化酶As(III)S-腺苷甲硫氨酸(SAM)甲基转移酶(AS3MT)的表达水平。我们表明,MAs(III)对神经元的毒性显着高于As(III)。有趣的是,细胞类型之间的细胞毒性差异不是由于AS3MT的表达引起的,因为它在神经元和神经胶质中表达,但在内皮细胞中不表达。这些结果支持了我们的假设,即MAs(III)是可能的生理神经毒素,而不是无机砷。
更新日期:2020-02-12
中文翻译:

无机亚砷酸盐和甲基亚砷酸盐在人脑细胞中的比较细胞毒性。
这项研究的总体目标是阐明砷对多种人脑细胞的潜在作用。砷是最普遍的人类A类环境致癌物。长期接触砷与人类疾病有关,包括癌症,心血管疾病和糖尿病。对子宫内砷暴露的婴幼儿神经发育和相关疾病的健康影响更为直接。砷在各种器官和组织中代谢为毒性更大的甲基化物质,包括甲基亚砷酸盐(MAs(III)),因此出现了一个问题,即甲基化物质是否对砷的神经系统作用负责。砷通过血脑屏障进入大脑,并在大脑微血管内皮细胞,神经胶质细胞(星形胶质细胞和小神经胶质细胞)和神经元中产生毒性。在这项研究中,我们首先使用基于形态学和细胞毒性的分析方法评估了暴露于无机三价As(III)或MAs(III)的不同类型脑细胞的毒性。其次,我们确定了几种类型的脑细胞中砷的甲基化以及甲基化酶As(III)S-腺苷甲硫氨酸(SAM)甲基转移酶(AS3MT)的表达水平。我们表明,MAs(III)对神经元的毒性显着高于As(III)。有趣的是,细胞类型之间的细胞毒性差异不是由于AS3MT的表达,因为它在神经元和神经胶质中表达,但在内皮细胞中不表达。这些结果支持了我们的假设,即MAs(III)是可能的生理神经毒素,而不是无机砷。我们首先使用基于形态学和细胞毒性的分析方法评估了暴露于无机三价As(III)或MAs(III)的不同类型脑细胞的毒性。其次,我们确定了几种类型的脑细胞中砷的甲基化以及甲基化酶As(III)S-腺苷甲硫氨酸(SAM)甲基转移酶(AS3MT)的表达水平。我们表明,MAs(III)对神经元的毒性显着高于As(III)。有趣的是,细胞类型之间的细胞毒性差异不是由于AS3MT的表达,因为它在神经元和神经胶质中表达,但在内皮细胞中不表达。这些结果支持了我们的假设,即MAs(III)是可能的生理神经毒素,而不是无机砷。我们首先使用基于形态学和细胞毒性的分析方法评估了暴露于无机三价As(III)或MAs(III)的不同类型脑细胞的毒性。其次,我们确定了几种类型的脑细胞中砷的甲基化以及甲基化酶As(III)S-腺苷甲硫氨酸(SAM)甲基转移酶(AS3MT)的表达水平。我们表明,MAs(III)对神经元的毒性显着高于As(III)。有趣的是,细胞类型之间的细胞毒性差异不是由于AS3MT的表达引起的,因为它在神经元和神经胶质中表达,但在内皮细胞中不表达。这些结果支持了我们的假设,即MAs(III)是可能的生理神经毒素,而不是无机砷。









































 京公网安备 11010802027423号
京公网安备 11010802027423号