当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, crystal structure, and ADME prediction studies of novel imidazopyrimidines as antibacterial and cytotoxic agents
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-01-27 , DOI: 10.1002/ardp.201900271 Heba T Abdel-Mohsen 1 , Amira Abood 1 , Keith J Flanagan 2 , Alina Meindl 2 , Mathias O Senge 2 , Hoda I El Diwani 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-01-27 , DOI: 10.1002/ardp.201900271 Heba T Abdel-Mohsen 1 , Amira Abood 1 , Keith J Flanagan 2 , Alina Meindl 2 , Mathias O Senge 2 , Hoda I El Diwani 1
Affiliation
|
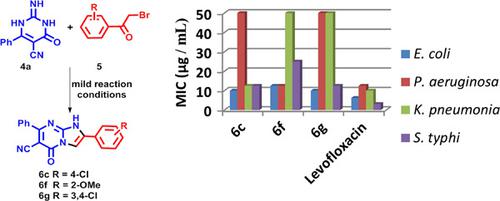
In the present study, a novel series of polyfunctionalized imidazopyrimidines 6a–u and 9a–d were efficiently constructed by a domino reaction between 2‐imino‐6‐substituted‐2,3‐dihydropyrimidin‐4(1H)‐ones 4a–d or 8a–c and 2‐bromoacetophenones 5a–i under mild basic conditions. The synthesized series were screened for their antibacterial activity against Staphylococcus aureus and Bacillus subtilis as Gram‐positive (+) bacteria, as well as against Gram‐negative (−) bacteria Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Salmonella typhi. Most of the synthesized derivatives of imidazopyrimidines 6 and 9 showed remarkable selectivity against Gram(−) bacteria over the Gram(+) ones. Compounds 6c, 6f, and 6g displayed potent and broad‐spectrum antibacterial activity against all tested strains. Compounds 6f and 6g displayed promising inhibitory activity on GryB ATPase from E. coli with IC50 = 1.14 and 0.73 μM, respectively. Simultaneously, some of the synthesized imidazopyrimidines were screened for their antiproliferative activity against 60 cancer cell lines at a concentration of 10 μM. Compound 9d showed potent activity against most of the tested cell lines, with a mean growth inhibition of 37%. The ADME (absorption, distribution, metabolism, and excretion) prediction study demonstrated that the synthesized hits have, in addition to their promising chemotherapeutic activity, acceptable pharmacokinetic properties, and a drug‐likeness nature to be further developed.
中文翻译:

作为抗菌剂和细胞毒剂的新型咪唑并嘧啶的合成、晶体结构和 ADME 预测研究
在本研究中,通过 2-亚氨基-6-取代-2,3-二氢嘧啶-4(1H)-酮 4a-d 或8a-c 和 2-溴苯乙酮 5a-i 在温和的碱性条件下。筛选合成的系列对金黄色葡萄球菌和枯草芽孢杆菌作为革兰氏阳性 (+) 细菌以及革兰氏阴性 (-) 细菌大肠杆菌、肺炎克雷伯菌、铜绿假单胞菌和伤寒沙门氏菌的抗菌活性。大多数合成的咪唑并嘧啶衍生物 6 和 9 对革兰 (-) 细菌显示出显着的选择性,而不是对革兰 (+) 细菌。化合物 6c、6f 和 6g 对所有测试菌株均显示出有效的广谱抗菌活性。化合物 6f 和 6g 对来自大肠杆菌的 GryB ATPase 显示出有希望的抑制活性,IC50 分别为 1.14 和 0.73 μM。同时,在 10 μM 的浓度下,筛选了一些合成的咪唑并嘧啶对 60 种癌细胞系的抗增殖活性。化合物 9d 显示出对大多数测试细胞系的有效活性,平均生长抑制率为 37%。ADME(吸收、分布、代谢和排泄)预测研究表明,合成的命中物除了具有有希望的化疗活性、可接受的药代动力学特性以及有待进一步开发的药物相似性外,还具有。在 10 μM 的浓度下,筛选了一些合成的咪唑并嘧啶对 60 种癌细胞系的抗增殖活性。化合物 9d 显示出对大多数测试细胞系的有效活性,平均生长抑制率为 37%。ADME(吸收、分布、代谢和排泄)预测研究表明,合成的命中物除了具有有希望的化疗活性、可接受的药代动力学特性以及有待进一步开发的药物相似性外,还具有。在 10 μM 的浓度下,筛选了一些合成的咪唑并嘧啶对 60 种癌细胞系的抗增殖活性。化合物 9d 显示出对大多数测试细胞系的有效活性,平均生长抑制率为 37%。ADME(吸收、分布、代谢和排泄)预测研究表明,合成的命中物除了具有有前景的化疗活性、可接受的药代动力学特性外,还具有有待进一步开发的药物相似性。
更新日期:2020-01-27
中文翻译:

作为抗菌剂和细胞毒剂的新型咪唑并嘧啶的合成、晶体结构和 ADME 预测研究
在本研究中,通过 2-亚氨基-6-取代-2,3-二氢嘧啶-4(1H)-酮 4a-d 或8a-c 和 2-溴苯乙酮 5a-i 在温和的碱性条件下。筛选合成的系列对金黄色葡萄球菌和枯草芽孢杆菌作为革兰氏阳性 (+) 细菌以及革兰氏阴性 (-) 细菌大肠杆菌、肺炎克雷伯菌、铜绿假单胞菌和伤寒沙门氏菌的抗菌活性。大多数合成的咪唑并嘧啶衍生物 6 和 9 对革兰 (-) 细菌显示出显着的选择性,而不是对革兰 (+) 细菌。化合物 6c、6f 和 6g 对所有测试菌株均显示出有效的广谱抗菌活性。化合物 6f 和 6g 对来自大肠杆菌的 GryB ATPase 显示出有希望的抑制活性,IC50 分别为 1.14 和 0.73 μM。同时,在 10 μM 的浓度下,筛选了一些合成的咪唑并嘧啶对 60 种癌细胞系的抗增殖活性。化合物 9d 显示出对大多数测试细胞系的有效活性,平均生长抑制率为 37%。ADME(吸收、分布、代谢和排泄)预测研究表明,合成的命中物除了具有有希望的化疗活性、可接受的药代动力学特性以及有待进一步开发的药物相似性外,还具有。在 10 μM 的浓度下,筛选了一些合成的咪唑并嘧啶对 60 种癌细胞系的抗增殖活性。化合物 9d 显示出对大多数测试细胞系的有效活性,平均生长抑制率为 37%。ADME(吸收、分布、代谢和排泄)预测研究表明,合成的命中物除了具有有希望的化疗活性、可接受的药代动力学特性以及有待进一步开发的药物相似性外,还具有。在 10 μM 的浓度下,筛选了一些合成的咪唑并嘧啶对 60 种癌细胞系的抗增殖活性。化合物 9d 显示出对大多数测试细胞系的有效活性,平均生长抑制率为 37%。ADME(吸收、分布、代谢和排泄)预测研究表明,合成的命中物除了具有有前景的化疗活性、可接受的药代动力学特性外,还具有有待进一步开发的药物相似性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号