当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of 4,4-(Difluoro)glutamic Acid via Chiral Ni(II)-Complexes of Dehydroalanine Schiff Bases. Effect of the Chiral Ligands Structure on the Stereochemical Outcome.
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-01-29 , DOI: 10.1002/open.201900343 Yoshinori Tokairin 1 , Yuhei Shigeno 2 , Jianlin Han 3 , Gerd-Volker Röschenthaler 1 , Hiroyuki Konno 2 , Hiroki Moriwaki 4 , Vadim A Soloshonok 5, 6
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-01-29 , DOI: 10.1002/open.201900343 Yoshinori Tokairin 1 , Yuhei Shigeno 2 , Jianlin Han 3 , Gerd-Volker Röschenthaler 1 , Hiroyuki Konno 2 , Hiroki Moriwaki 4 , Vadim A Soloshonok 5, 6
Affiliation
|
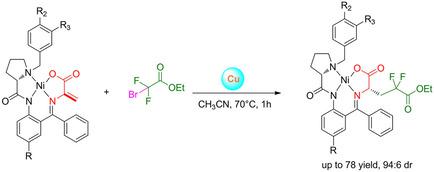
Four differently substituted chiral Ni(II)‐complexes of dehydroalanine Schiff base were prepared and reacted with BrCF2COOEt/Cu under the standard reaction conditions. The observed diastereoselectivity was found to depend on the degree and pattern of chlorine substitution for hydrogen in the structure of the dehydroalanine complexes. The unsubstituted complex gave the ratio of diastereomers (S)(2S)/(S)(2R) of 66/34. On the other hand, introduction of chlorine atoms in the strategic positions on the chiral ligands allowed to achieve a practically attractive diastereoselectivity of (∼98.5/1.5). Diastereomerically pure major product was disassembled to prepare 9‐fluorenylmethyloxycarbonyl (Fmoc) derivative of (S)‐4,4‐difluoroglutamic acid.
中文翻译:

通过脱氢丙氨酸席夫碱的手性 Ni(II) 配合物不对称合成 4,4-(二氟)谷氨酸。手性配体结构对立体化学结果的影响。
制备了四种不同取代的脱氢丙氨酸席夫碱手性Ni(II)配合物,并在标准反应条件下与BrCF 2 COOEt/Cu反应。发现观察到的非对映选择性取决于脱氢丙氨酸复合物结构中氯取代氢的程度和模式。未取代的复合物的非对映异构体( S )( 2S )/( S )( 2R )之比为66/34。另一方面,在手性配体的战略位置引入氯原子可以实现具有实际吸引力的非对映选择性(~98.5/1.5)。将非对映体纯的主要产物分解,制备( S )-4,4-二氟谷氨酸的9-芴基甲氧基羰基(Fmoc)衍生物。
更新日期:2020-01-29
中文翻译:

通过脱氢丙氨酸席夫碱的手性 Ni(II) 配合物不对称合成 4,4-(二氟)谷氨酸。手性配体结构对立体化学结果的影响。
制备了四种不同取代的脱氢丙氨酸席夫碱手性Ni(II)配合物,并在标准反应条件下与BrCF 2 COOEt/Cu反应。发现观察到的非对映选择性取决于脱氢丙氨酸复合物结构中氯取代氢的程度和模式。未取代的复合物的非对映异构体( S )( 2S )/( S )( 2R )之比为66/34。另一方面,在手性配体的战略位置引入氯原子可以实现具有实际吸引力的非对映选择性(~98.5/1.5)。将非对映体纯的主要产物分解,制备( S )-4,4-二氟谷氨酸的9-芴基甲氧基羰基(Fmoc)衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号