当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Silver(I)‐Catalyzed Regioselective Synthesis of Dihydrofuro[3,4‐b]quinolines from o‐Alkynylquinoline‐MBH Adducts and Evaluation of their Photophysical Properties
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-02-07 , DOI: 10.1002/ajoc.202000002 Vipin Kumar 1 , Sandip Kumar Tiwari 2 , Virender Singh 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-02-07 , DOI: 10.1002/ajoc.202000002 Vipin Kumar 1 , Sandip Kumar Tiwari 2 , Virender Singh 1
Affiliation
|
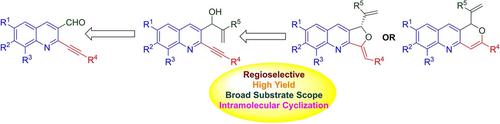
A simple and facile approach is described for the regioselective synthesis of dihydrofuro[3,4‐b]quinolines from ortho‐alkynylquinoline‐Morita‐Baylis‐Hillman adducts under mild reaction conditions. Interestingly, in case of aromatic alkynes, 5‐exo‐dig cyclization was observed, while in case of aliphatic long chain alkynes, 6‐endo‐dig cyclized products were obtained. The present approach does not require any dry conditions, tedious work‐up or inert atmosphere for the production of dihydrofuro[3,4‐b]quinolines. The exact structures and stereochemistry of the synthesized molecules were assigned by NMR and X‐ray crystallographic analysis. Their photophysical properties were also evaluated.
中文翻译:

银(I)催化的邻炔基喹啉-MBH加合物合成二氢呋喃[3,4-b]喹啉的区域选择性及其光物理性质的评价
描述了一种在温和的反应条件下从邻炔基喹啉-Morita-Baylis-Hillman加合物的区域选择性合成二氢呋喃并[3,4- b ]喹啉的简便方法。有趣的是,在芳香族炔烃的情况下,观察到了5酯基环化,而在脂肪族长链炔烃的情况下,得到了6酯基环化产物。本方法不需要任何干燥条件,繁琐的工作或惰性气氛即可生产二氢呋喃[3,4- b ]喹啉。通过NMR和X射线晶体学分析确定了合成分子的确切结构和立体化学。还评估了它们的光物理性质。
更新日期:2020-04-21
中文翻译:

银(I)催化的邻炔基喹啉-MBH加合物合成二氢呋喃[3,4-b]喹啉的区域选择性及其光物理性质的评价
描述了一种在温和的反应条件下从邻炔基喹啉-Morita-Baylis-Hillman加合物的区域选择性合成二氢呋喃并[3,4- b ]喹啉的简便方法。有趣的是,在芳香族炔烃的情况下,观察到了5酯基环化,而在脂肪族长链炔烃的情况下,得到了6酯基环化产物。本方法不需要任何干燥条件,繁琐的工作或惰性气氛即可生产二氢呋喃[3,4- b ]喹啉。通过NMR和X射线晶体学分析确定了合成分子的确切结构和立体化学。还评估了它们的光物理性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号