Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Characterization of Tau in Fuzzy Tau:Tubulin Complexes.
Structure ( IF 4.4 ) Pub Date : 2020-01-28 , DOI: 10.1016/j.str.2020.01.004 Ho Yee Joyce Fung 1 , Kristen M McKibben 2 , Jennifer Ramirez 2 , Kushol Gupta 3 , Elizabeth Rhoades 4
Structure ( IF 4.4 ) Pub Date : 2020-01-28 , DOI: 10.1016/j.str.2020.01.004 Ho Yee Joyce Fung 1 , Kristen M McKibben 2 , Jennifer Ramirez 2 , Kushol Gupta 3 , Elizabeth Rhoades 4
Affiliation
|
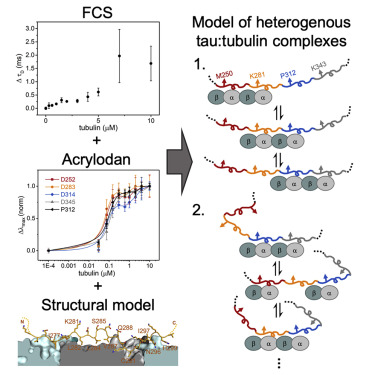
Tau is a neuronal microtubule (MT)-associated protein of significant interest due to its association with several neurodegenerative disorders. Tau's intrinsic disorder and the dynamic nature of its interactions with tubulin and MTs make its structural characterization challenging. Here, we use an environmentally sensitive fluorophore as a site-specific probe of tau bound to soluble tubulin. Comparison of our results with a recently published tau:MT cryoelectron microscopy model reveals structural similarities between tubulin- and MT-bound tau. Analysis of residues across the repeat regions reveals a hierarchy in tubulin occupancy, which may be relevant to tau's ability to differentiate between tubulin and MTs. As binding to soluble tubulin is a critical first step in MT polymerization, our characterization of the structural features of tau in dynamic, fuzzy tau:tubulin assemblies advances our understanding of how tau functions in the cell and how function may be disrupted in disease.
中文翻译:

模糊 Tau 中 Tau 的结构表征:微管蛋白复合物。
Tau 是一种具有重要意义的神经元微管 (MT) 相关蛋白,因为它与几种神经退行性疾病有关。Tau 的内在紊乱及其与微管蛋白和 MT 相互作用的动态特性使其结构表征具有挑战性。在这里,我们使用环境敏感的荧光团作为与可溶性微管蛋白结合的 tau 的位点特异性探针。我们的结果与最近发表的 tau:MT 冷冻电子显微镜模型的比较揭示了微管蛋白和 MT 结合的 tau 之间的结构相似性。对重复区域残基的分析揭示了微管蛋白占据的层次结构,这可能与 tau 区分微管蛋白和 MT 的能力有关。由于与可溶性微管蛋白结合是 MT 聚合的关键第一步,
更新日期:2020-01-29
中文翻译:

模糊 Tau 中 Tau 的结构表征:微管蛋白复合物。
Tau 是一种具有重要意义的神经元微管 (MT) 相关蛋白,因为它与几种神经退行性疾病有关。Tau 的内在紊乱及其与微管蛋白和 MT 相互作用的动态特性使其结构表征具有挑战性。在这里,我们使用环境敏感的荧光团作为与可溶性微管蛋白结合的 tau 的位点特异性探针。我们的结果与最近发表的 tau:MT 冷冻电子显微镜模型的比较揭示了微管蛋白和 MT 结合的 tau 之间的结构相似性。对重复区域残基的分析揭示了微管蛋白占据的层次结构,这可能与 tau 区分微管蛋白和 MT 的能力有关。由于与可溶性微管蛋白结合是 MT 聚合的关键第一步,











































 京公网安备 11010802027423号
京公网安备 11010802027423号