Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Function Analyses of a Keratin Heterotypic Complex Identify Specific Keratin Regions Involved in Intermediate Filament Assembly.
Structure ( IF 4.4 ) Pub Date : 2020-01-24 , DOI: 10.1016/j.str.2020.01.002 Chang-Hun Lee 1 , Min-Sung Kim 2 , Shuang Li 1 , Daniel J Leahy 3 , Pierre A Coulombe 4
Structure ( IF 4.4 ) Pub Date : 2020-01-24 , DOI: 10.1016/j.str.2020.01.002 Chang-Hun Lee 1 , Min-Sung Kim 2 , Shuang Li 1 , Daniel J Leahy 3 , Pierre A Coulombe 4
Affiliation
|
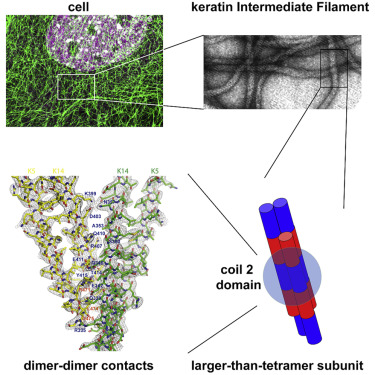
Intermediate filaments (IFs) provide vital mechanical support in a broad array of cell types. Interference with this role causes cell fragility and accounts for a large number of human diseases. Gaining an understanding of the structure of IFs is paramount to understanding their function and designing therapeutic agents for relevant diseases. Here, we report the 2.6-Å resolution crystal structure of a complex of interacting 2B domains of keratin 5 (K5) and K14. K5 and K14 form a long-range, left-handed coiled coil, with participating α helices aligned in parallel and in register. Follow-up mutagenesis revealed that specific contacts between interacting 2B domains play a crucial role during 10-nm IF assembly, likely at the step of octamer-octamer association. The resulting structural model represents an atomic-resolution visualization of 2B-2B interactions important to filament assembly and provides insight into the defects introduced by mutations in IF genes associated with human skin diseases.
中文翻译:

角蛋白异型复合物的结构功能分析确定了涉及中间丝组装的特定角蛋白区域。
中间丝(IF)在各种各样的细胞类型中提供了至关重要的机械支持。干扰此作用会导致细胞脆弱,并导致大量人类疾病。了解IFs的结构对于理解其功能和设计相关疾病的治疗剂至关重要。在这里,我们报告了角蛋白5(K5)和K14相互作用2B域的复合物的2.6-Å分辨率晶体结构。K5和K14形成一个远程左旋线圈,参与的α螺旋平行对齐并对齐。后续诱变显示,相互作用的2B域之间的特定接触在10 nm IF组装过程中起着至关重要的作用,可能是在八聚体-八聚体缔合的步骤中。
更新日期:2020-01-29
中文翻译:

角蛋白异型复合物的结构功能分析确定了涉及中间丝组装的特定角蛋白区域。
中间丝(IF)在各种各样的细胞类型中提供了至关重要的机械支持。干扰此作用会导致细胞脆弱,并导致大量人类疾病。了解IFs的结构对于理解其功能和设计相关疾病的治疗剂至关重要。在这里,我们报告了角蛋白5(K5)和K14相互作用2B域的复合物的2.6-Å分辨率晶体结构。K5和K14形成一个远程左旋线圈,参与的α螺旋平行对齐并对齐。后续诱变显示,相互作用的2B域之间的特定接触在10 nm IF组装过程中起着至关重要的作用,可能是在八聚体-八聚体缔合的步骤中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号