当前位置:
X-MOL 学术
›
Neurobiol. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Region-specific glial homeostatic signature in prion diseases is replaced by a uniform neuroinflammation signature, common for brain regions and prion strains with different cell tropism.
Neurobiology of Disease ( IF 5.1 ) Pub Date : 2020-01-27 , DOI: 10.1016/j.nbd.2020.104783 Natallia Makarava 1 , Jennifer Chen-Yu Chang 1 , Kara Molesworth 1 , Ilia V Baskakov 1
Neurobiology of Disease ( IF 5.1 ) Pub Date : 2020-01-27 , DOI: 10.1016/j.nbd.2020.104783 Natallia Makarava 1 , Jennifer Chen-Yu Chang 1 , Kara Molesworth 1 , Ilia V Baskakov 1
Affiliation
|
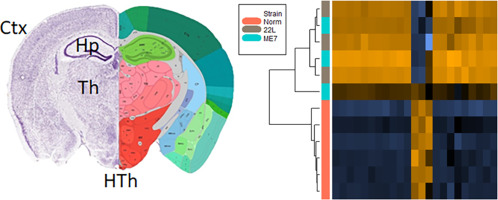
Chronic neuroinflammation is recognized as a major neuropathological hallmark in a broad spectrum of neurodegenerative diseases including Alzheimer's, Parkinson's, Frontal Temporal Dementia, Amyotrophic Lateral Sclerosis, and prion diseases. Both microglia and astrocytes exhibit region-specific homeostatic transcriptional identities, which under chronic neurodegeneration, transform into reactive phenotypes in a region- and disease-specific manner. Little is known about region-specific identity of glia in prion diseases. The current study was designed to determine whether the region-specific homeostatic signature of glia changes with the progression of prion diseases, and whether these changes occur in a region-dependent or universal manner. Also of interest was whether different prion strains give rise to different reactive phenotypes. To answer these questions, we analyzed gene expression in the thalamus, cortex, hypothalamus and hippocampus of mice infected with 22L and ME7 prion strains using a Nanostring Neuroinflammation panel at the subclinical, early clinical and advanced stages of the disease. We found that at the preclinical stage of the disease, the region-specific homeostatic identities were preserved. However, with the appearance of clinical signs, the region-specific signatures were partially lost and replaced with a neuroinflammation signature. While the same sets of genes were activated by both prion strains, the timing of neuroinflammation and the degree of activation in different brain regions was strain-specific. Changes in astrocyte function scored at the top of the activated pathways. Moreover, clustering analysis suggested that the astrocyte function pathway responded to prion infection prior to the Activated Microglia or Neuron and Neurotransmission pathways. The current work established neuroinflammation gene expression signature associated with prion diseases. Our results illustrate that with the disease progression, the region-specific homeostatic transcriptome signatures are replaced by the region-independent neuroinflammation signature, which is common for prion strains with different cell tropism. The prion-associated neuroinflammation signature identified in the current study overlapped only partially with the microglia degenerative phenotype and the disease-associated microglia phenotype reported for animal models of other neurodegenerative diseases.
中文翻译:

病毒疾病中特定于区域的神经胶质稳态信号被统一的神经炎症信号所取代,这在大脑区域和具有不同细胞嗜性的病毒菌株中很常见。
慢性神经炎症被认为是广泛的神经退行性疾病的主要神经病理学标志,包括阿尔茨海默氏病,帕金森氏病,额颞痴呆,肌萎缩性侧索硬化症和病毒疾病。小胶质细胞和星形胶质细胞均表现出区域特异性的稳态转录身份,在慢性神经变性下,其以区域和疾病特异性的方式转变为反应性表型。关于病毒疾病中神经胶质的区域特定身份知之甚少。当前的研究旨在确定神经胶质的区域特定的稳态信号是否随of病毒疾病的发展而变化,以及这些变化是否以区域依赖或普遍的方式发生。同样令人感兴趣的是,不同的病毒菌株是否引起不同的反应性表型。为了回答这些问题,我们在疾病的亚临床阶段,早期临床阶段和晚期阶段,使用纳米线神经炎症小组分析了感染22L和ME7 pr病毒株的小鼠的丘脑,皮质,下丘脑和海马中的基因表达。我们发现,在该疾病的临床前阶段,保留了区域特定的稳态特性。然而,随着临床体征的出现,该区域特异性标志被部分丢失,并被神经炎症标志所代替。尽管两种病毒菌株都激活了相同的基因集,但神经炎症的时机和不同大脑区域的激活程度是菌株特异性的。星形胶质细胞功能的变化记录在激活途径的顶部。此外,聚类分析表明,星形胶质细胞功能途径先于激活的小胶质细胞或神经元和神经传递途径对病毒感染作出反应。目前的工作建立了与病毒疾病有关的神经炎症基因表达特征。我们的结果表明,随着疾病的进展,区域特异性的稳态转录组标记被区域独立的神经炎症标记所取代,这对于具有不同细胞嗜性的病毒菌株很常见。在本研究中确定的病毒相关的神经炎症特征仅与小胶质细胞变性表型和其他神经退行性疾病动物模型报道的与疾病相关的小胶质细胞表型重叠。
更新日期:2020-01-27
中文翻译:

病毒疾病中特定于区域的神经胶质稳态信号被统一的神经炎症信号所取代,这在大脑区域和具有不同细胞嗜性的病毒菌株中很常见。
慢性神经炎症被认为是广泛的神经退行性疾病的主要神经病理学标志,包括阿尔茨海默氏病,帕金森氏病,额颞痴呆,肌萎缩性侧索硬化症和病毒疾病。小胶质细胞和星形胶质细胞均表现出区域特异性的稳态转录身份,在慢性神经变性下,其以区域和疾病特异性的方式转变为反应性表型。关于病毒疾病中神经胶质的区域特定身份知之甚少。当前的研究旨在确定神经胶质的区域特定的稳态信号是否随of病毒疾病的发展而变化,以及这些变化是否以区域依赖或普遍的方式发生。同样令人感兴趣的是,不同的病毒菌株是否引起不同的反应性表型。为了回答这些问题,我们在疾病的亚临床阶段,早期临床阶段和晚期阶段,使用纳米线神经炎症小组分析了感染22L和ME7 pr病毒株的小鼠的丘脑,皮质,下丘脑和海马中的基因表达。我们发现,在该疾病的临床前阶段,保留了区域特定的稳态特性。然而,随着临床体征的出现,该区域特异性标志被部分丢失,并被神经炎症标志所代替。尽管两种病毒菌株都激活了相同的基因集,但神经炎症的时机和不同大脑区域的激活程度是菌株特异性的。星形胶质细胞功能的变化记录在激活途径的顶部。此外,聚类分析表明,星形胶质细胞功能途径先于激活的小胶质细胞或神经元和神经传递途径对病毒感染作出反应。目前的工作建立了与病毒疾病有关的神经炎症基因表达特征。我们的结果表明,随着疾病的进展,区域特异性的稳态转录组标记被区域独立的神经炎症标记所取代,这对于具有不同细胞嗜性的病毒菌株很常见。在本研究中确定的病毒相关的神经炎症特征仅与小胶质细胞变性表型和其他神经退行性疾病动物模型报道的与疾病相关的小胶质细胞表型重叠。











































 京公网安备 11010802027423号
京公网安备 11010802027423号