当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper selective separation and nanoporous copper oxide preparation from waste copper-clad aluminum scraps
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.hydromet.2020.105287 Shuang Shao , Baozhong Ma , Lei Zeng , Peng Xing , Bao Liu , Chengyan Wang
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.hydromet.2020.105287 Shuang Shao , Baozhong Ma , Lei Zeng , Peng Xing , Bao Liu , Chengyan Wang
|
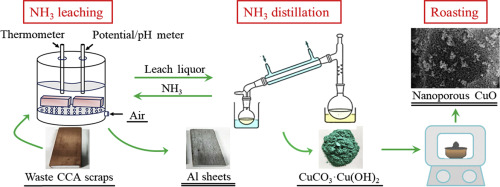
Abstract Waste copper-clad aluminum (CCA) scraps are secondary resources that are rich in Cu and Al, with biosafety disposal that bears considerable strategic significance in promoting CCA. A process comprising NH3 leaching, NH3 distillation, and roasting was proposed to separate Cu from waste CCA scraps and prepare nanoporous CuO. First, NH3 leaching was performed to separate Cu from scraps. The effects of initial Cu2+ concentration, NH3 concentration, and air flow rate on Cu leaching speed were assessed. Over 99% Cu could be selectively leached, whereas nearly no Al reacted in optimum conditions. Leaching mechanism was analyzed by combining the changes in the potential and pH of the entire NH3 leaching process. Subsequently, NH3 distillation of the leach liquor was carried out to prepare a precursor, which was confirmed to be CuCO3·Cu(OH)2 by X-ray powder diffraction (XRD) analysis. Finally, CuCO3·Cu(OH)2 was roasted, and the effect of roasting temperature on roasted product composition and properties was investigated by thermogravimetry–differential scanning calorimetry, XRD, scanning electron microscopy, and N2 adsorption–desorption. Results indicated that nanoporous CuO (purity, >99%; Brunauer–Emmett–Teller surface area, 44.38 m2/g; pore volume, 0.2690 cm3/g) was successfully prepared. In the entire process, 96.70% NH3 and 98% Cu were recovered via analytical calculation.
中文翻译:

从废铜包铝边角料中选择性分离铜及制备纳米多孔氧化铜
摘要 废铜包铝(CCA)边角料是富含铜、铝的二次资源,生物安全处置对推进CCA具有重要战略意义。提出了一种包括NH3浸出、NH3蒸馏和焙烧的工艺,以从废CCA废料中分离Cu并制备纳米多孔CuO。首先,进行NH3浸出以从废料中分离Cu。评估了初始 Cu2+ 浓度、NH3 浓度和空气流速对 Cu 浸出速度的影响。超过 99% 的 Cu 可以被选择性浸出,而在最佳条件下几乎没有 Al 发生反应。结合整个NH3浸出过程电位和pH值的变化分析浸出机理。随后,对浸出液进行NH3蒸馏以制备前驱体,通过X射线粉末衍射(XRD)分析证实为CuCO3·Cu(OH)2。最后,对CuCO3·Cu(OH)2进行焙烧,通过热重-差示扫描量热法、XRD、扫描电子显微镜和N2吸附-解吸研究了焙烧温度对焙烧产物组成和性质的影响。结果表明纳米多孔氧化铜(纯度,>99%;Brunauer-Emmett-Teller 表面积,44.38 m2/g;孔体积,0.2690 cm3/g)被成功制备。整个过程通过分析计算回收了96.70%的NH3和98%的Cu。和 N2 吸附-解吸。结果表明纳米多孔氧化铜(纯度,>99%;Brunauer-Emmett-Teller 表面积,44.38 m2/g;孔体积,0.2690 cm3/g)被成功制备。整个过程通过分析计算回收了96.70%的NH3和98%的Cu。和 N2 吸附-解吸。结果表明纳米多孔氧化铜(纯度,>99%;Brunauer-Emmett-Teller 表面积,44.38 m2/g;孔体积,0.2690 cm3/g)被成功制备。整个过程通过分析计算回收了96.70%的NH3和98%的Cu。
更新日期:2020-03-01
中文翻译:

从废铜包铝边角料中选择性分离铜及制备纳米多孔氧化铜
摘要 废铜包铝(CCA)边角料是富含铜、铝的二次资源,生物安全处置对推进CCA具有重要战略意义。提出了一种包括NH3浸出、NH3蒸馏和焙烧的工艺,以从废CCA废料中分离Cu并制备纳米多孔CuO。首先,进行NH3浸出以从废料中分离Cu。评估了初始 Cu2+ 浓度、NH3 浓度和空气流速对 Cu 浸出速度的影响。超过 99% 的 Cu 可以被选择性浸出,而在最佳条件下几乎没有 Al 发生反应。结合整个NH3浸出过程电位和pH值的变化分析浸出机理。随后,对浸出液进行NH3蒸馏以制备前驱体,通过X射线粉末衍射(XRD)分析证实为CuCO3·Cu(OH)2。最后,对CuCO3·Cu(OH)2进行焙烧,通过热重-差示扫描量热法、XRD、扫描电子显微镜和N2吸附-解吸研究了焙烧温度对焙烧产物组成和性质的影响。结果表明纳米多孔氧化铜(纯度,>99%;Brunauer-Emmett-Teller 表面积,44.38 m2/g;孔体积,0.2690 cm3/g)被成功制备。整个过程通过分析计算回收了96.70%的NH3和98%的Cu。和 N2 吸附-解吸。结果表明纳米多孔氧化铜(纯度,>99%;Brunauer-Emmett-Teller 表面积,44.38 m2/g;孔体积,0.2690 cm3/g)被成功制备。整个过程通过分析计算回收了96.70%的NH3和98%的Cu。和 N2 吸附-解吸。结果表明纳米多孔氧化铜(纯度,>99%;Brunauer-Emmett-Teller 表面积,44.38 m2/g;孔体积,0.2690 cm3/g)被成功制备。整个过程通过分析计算回收了96.70%的NH3和98%的Cu。











































 京公网安备 11010802027423号
京公网安备 11010802027423号