当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strategies toward Aryne Multifunctionalization via 1,2-Benzdiyne and Benzyne.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-01-27 , DOI: 10.1021/acs.accounts.9b00608 Jia He 1 , Dachuan Qiu 1 , Yang Li 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-01-27 , DOI: 10.1021/acs.accounts.9b00608 Jia He 1 , Dachuan Qiu 1 , Yang Li 1
Affiliation
|
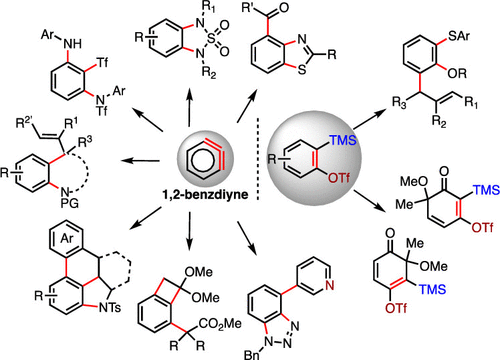
Polysubstituted arenes are prevalent in numerous natural products, medicines, agrochemicals, and organic functional materials. Among methods to prepare polysubstituted arenes, pathways involving benzyne intermediates are particularly attractive given they can readily assemble highly diverse vicinal difunctionalized benzenes in a step-economical manner under transition-metal-free conditions. In order to incorporate more than two substituents on a benzene ring via a benzyne intermediate, methodologies involving benzdiyne and benztriyne have been developed, which significantly expand the current difunctionalization strategies in benzyne chemistry. In the past years, our group has been focusing on pushing the frontier of traditional benzyne chemistry and exploring new applications. In an aim to efficiently and conveniently construct polysubstituted arenes, we developed several aryne multifunctionalization strategies. The first strategy is through the 1,2-benzdiyne processes, which can be divided into a domino aryne approach and stepwise 1,2-benzdiyne approach. In our domino aryne study, we developed three domino aryne reagents as "sesquibenzyne" synthons, which are complementary in terms of reactivity and could adapt different modes of cascade transformations. By employing these domino aryne precursors, we were able to accomplish several cascade transformations, including double nucleophilic reactions, i.e., the reaction with carbonyl protected benzothioamides, 1,2-diamination, and 1,3-diamination. In addition, two cascade processes involving nucleophilic and pericyclic reactions, namely, domino aryne annulation via nucleophilic-ene cascade and domino aryne nucleophilic, Diels-Alder process, were successfully achieved as well. Meanwhile, with our desire to expand the scope of 1,2-benzdiyne transformations, we employed stepwise 1,2-benzdiyne tactics to access polysubstituted arenes. Depending on the property of the substituent on the C3-position of a benzyne intermediate, either an electron-withdrawing or electron-donating group, the incoming ortho groups were chosen accordingly. Consequently, we realized a [2 + 2] cycloaddition-Grob fragmentation process using 3-triflyloxybenzyne and a 1,2-benzdiyne process using 3-(trimethylsilyl)benzyne. Our second research strategy is to use single benzyne to access trisubstituted arenes, which represents a more atom- and step-economical protocol. With our deliberate design, we discovered a tandem benzyne S═O bond insertion/C-H functionalization process using single benzyne and aryl allyl sulfoxides, furnishing three chemical bonds of different types in a single process. This transformation is the first 1,2,3-trisubstitution example using single benzyne intermediate. At last, we developed an oxidative dearomatization strategy on Kobayashi benzyne precursors, which led to the preparation of various cyclohexenynone precursors with diverse substituents in highly efficient manner. In this study, we also demonstrated a new reaction mode of these cyclohexenynones with allyl sulfoxides, which involves a deeper utilization of the cyclohexyne triple bond. This work is the first example of successful connection of precursors of benzyne with cyclohexyne together, revealing a new research direction in the field of cyclohexyne.
中文翻译:

通过1,2-Benzdiyne和Benzyne实现Aryne多功能化的策略。
多取代的芳烃普遍存在于许多天然产物,药物,农用化学品和有机功能材料中。在制备多取代的芳烃的方法中,涉及苯并中间体的途径是特别有吸引力的,因为它们可以在无过渡金属的条件下以逐步经济的方式容易地组装高度多样的邻位双官能化苯。为了通过苯并炔中间体在苯环上结合两个以上的取代基,已经开发出涉及苯并二炔和苯并三炔的方法,这大大扩展了当前苯并炔化学中的双官能化策略。在过去的几年中,我们小组一直致力于推动传统苯并炔化学的前沿和探索新的应用。为了有效和方便地构建多取代的芳烃,我们开发了几种芳烃多功能化策略。第一种策略是通过1,2-苯并二炔过程,该过程可以分为多米诺骨牌方法和逐步1,2-苯并二炔方法。在我们的多米诺骨牌研究中,我们开发了三种多米诺骨牌试剂作为“倍半苄”合成子,它们在反应性方面是互补的,可以适应不同的级联转化方式。通过使用这些多米诺烷芳烃前体,我们能够完成一些级联转化,包括双亲核反应,即与羰基保护的苯并硫代酰胺的反应,1,2-氨基化和1,3-氨基化。另外,涉及亲核反应和周环反应的两个级联过程,即 还成功实现了通过亲核烯级联的多米诺亚芳烷基环化反应和Diels-Alder工艺实现的多米诺亚芳基亲核反应。同时,由于我们希望扩大1,2-苯并二炔转化的范围,我们采用了逐步的1,2-苯并二炔策略来获得多取代的芳烃。根据吸电子基团或供电子基团在苯炔中间体的C 3-位上的取代基的性质,相应地选择进入的邻位基团。因此,我们实现了使用3-triflyloxybenzyne的[2 + 2]环加成-Grob裂解过程和使用3-(trimethylsilyl)benzyne的1,2-benzdiyne过程。我们的第二个研究策略是使用单苯并芳烃来访问三取代的芳烃,这代表了一种更加原子经济和阶梯经济的方案。通过我们精心的设计,我们发现了使用单个苯炔和芳基烯丙基亚砜的串联苯并S═O键插入/ CH功能化过程,在单个过程中提供了三种不同类型的化学键。该转化是使用单个苯炔中间体的第一个1,2,3-三取代实例。最后,我们开发了一种小林苯并炔前体的氧化脱芳香化策略,从而以高效的方式制备了各种具有不同取代基的环己酮前体。在这项研究中,我们还证明了这些环己烯酮与烯丙基亚砜的新反应模式,涉及更深地利用环己炔三键。这项工作是苯并炔与环己炔前体成功连接的第一个例子,揭示了环己炔领域的新研究方向。
更新日期:2020-01-27
中文翻译:

通过1,2-Benzdiyne和Benzyne实现Aryne多功能化的策略。
多取代的芳烃普遍存在于许多天然产物,药物,农用化学品和有机功能材料中。在制备多取代的芳烃的方法中,涉及苯并中间体的途径是特别有吸引力的,因为它们可以在无过渡金属的条件下以逐步经济的方式容易地组装高度多样的邻位双官能化苯。为了通过苯并炔中间体在苯环上结合两个以上的取代基,已经开发出涉及苯并二炔和苯并三炔的方法,这大大扩展了当前苯并炔化学中的双官能化策略。在过去的几年中,我们小组一直致力于推动传统苯并炔化学的前沿和探索新的应用。为了有效和方便地构建多取代的芳烃,我们开发了几种芳烃多功能化策略。第一种策略是通过1,2-苯并二炔过程,该过程可以分为多米诺骨牌方法和逐步1,2-苯并二炔方法。在我们的多米诺骨牌研究中,我们开发了三种多米诺骨牌试剂作为“倍半苄”合成子,它们在反应性方面是互补的,可以适应不同的级联转化方式。通过使用这些多米诺烷芳烃前体,我们能够完成一些级联转化,包括双亲核反应,即与羰基保护的苯并硫代酰胺的反应,1,2-氨基化和1,3-氨基化。另外,涉及亲核反应和周环反应的两个级联过程,即 还成功实现了通过亲核烯级联的多米诺亚芳烷基环化反应和Diels-Alder工艺实现的多米诺亚芳基亲核反应。同时,由于我们希望扩大1,2-苯并二炔转化的范围,我们采用了逐步的1,2-苯并二炔策略来获得多取代的芳烃。根据吸电子基团或供电子基团在苯炔中间体的C 3-位上的取代基的性质,相应地选择进入的邻位基团。因此,我们实现了使用3-triflyloxybenzyne的[2 + 2]环加成-Grob裂解过程和使用3-(trimethylsilyl)benzyne的1,2-benzdiyne过程。我们的第二个研究策略是使用单苯并芳烃来访问三取代的芳烃,这代表了一种更加原子经济和阶梯经济的方案。通过我们精心的设计,我们发现了使用单个苯炔和芳基烯丙基亚砜的串联苯并S═O键插入/ CH功能化过程,在单个过程中提供了三种不同类型的化学键。该转化是使用单个苯炔中间体的第一个1,2,3-三取代实例。最后,我们开发了一种小林苯并炔前体的氧化脱芳香化策略,从而以高效的方式制备了各种具有不同取代基的环己酮前体。在这项研究中,我们还证明了这些环己烯酮与烯丙基亚砜的新反应模式,涉及更深地利用环己炔三键。这项工作是苯并炔与环己炔前体成功连接的第一个例子,揭示了环己炔领域的新研究方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号