当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Guided Design of a Peptide Lock for Modular Peptide Binders.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-02-13 , DOI: 10.1021/acschembio.9b00928 Patrick Ernst 1 , Franziska Zosel 1 , Christian Reichen 1 , Daniel Nettels 1 , Benjamin Schuler 1 , Andreas Plückthun 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-02-13 , DOI: 10.1021/acschembio.9b00928 Patrick Ernst 1 , Franziska Zosel 1 , Christian Reichen 1 , Daniel Nettels 1 , Benjamin Schuler 1 , Andreas Plückthun 1
Affiliation
|
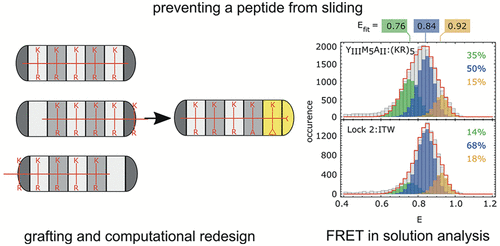
Peptides play an important role in intermolecular interactions and are frequent analytes in diagnostic assays, also as unstructured, linear epitopes in whole proteins. Yet, due to the many different sequence possibilities even for short peptides, classical selection of binding proteins from a library, one at a time, is not scalable to proteomes. However, moving away from selection to a rational assembly of preselected modules binding to predefined linear epitopes would split the problem into smaller parts. These modules could then be reassembled in any desired order to bind to, in principle, arbitrary sequences, thereby circumventing any new rounds of selection. Designed Armadillo repeat proteins (dArmRPs) are modular, and they do bind elongated peptides in a modular way. Their consensus sequence carries pockets that prefer arginine and lysine. In our quest to select pockets for all amino acid side chains, we had discovered that repetitive sequences can lead to register shifts and peptide flipping during selections from libraries, hindering the selection of new binding specificities. To solve this problem, we now created an orthogonal binding specificity by a combination of grafting from β-catenin, computational design and mutual optimization of the pocket and the bound peptide. We have confirmed the design and the desired interactions by X-ray structure determination. Furthermore, we could confirm the absence of sliding in solution by a single-molecule Förster resonance energy transfer. The new pocket could be moved from the N-terminus of the protein to the middle, retaining its properties, further underlining the modularity of the system.
中文翻译:

模块化肽结合剂的肽锁的结构导向设计。
肽在分子间的相互作用中起着重要的作用,并且在诊断分析中是常见的分析物,在整个蛋白质中也是非结构化的线性表位。然而,由于即使对于短肽而言也有许多不同的序列可能性,因此从文库中一次一次选择结合蛋白的经典选择不能扩展到蛋白质组学。但是,从选择转向绑定到预定义线性表位的预选模块的合理组装,会将问题分解为更小的部分。这些模块然后可以以任何期望的顺序重新组装,以原则上绑定到任意序列,从而避免了任何新的选择。设计的犰狳重复蛋白(dArmRPs)是模块化的,并且它们确实以模块化的方式结合细长的肽。他们的共有序列带有偏好精氨酸和赖氨酸的口袋。在为所有氨基酸侧链选择口袋的过程中,我们发现重复序列会导致在从文库中进行选择的过程中导致寄存器移位和肽翻转,从而阻碍了对新结合特异性的选择。为了解决这个问题,我们现在通过结合β-catenin的接枝,计算设计以及口袋和结合肽的相互优化来创建正交结合特异性。通过X射线结构确定,我们已经确认了设计和所需的相互作用。此外,我们可以通过单分子Förster共振能量转移确认溶液中不存在滑动。新的口袋可以从蛋白质的N端移到中间,保留其特性,从而进一步突出了系统的模块化。
更新日期:2020-02-14
中文翻译:

模块化肽结合剂的肽锁的结构导向设计。
肽在分子间的相互作用中起着重要的作用,并且在诊断分析中是常见的分析物,在整个蛋白质中也是非结构化的线性表位。然而,由于即使对于短肽而言也有许多不同的序列可能性,因此从文库中一次一次选择结合蛋白的经典选择不能扩展到蛋白质组学。但是,从选择转向绑定到预定义线性表位的预选模块的合理组装,会将问题分解为更小的部分。这些模块然后可以以任何期望的顺序重新组装,以原则上绑定到任意序列,从而避免了任何新的选择。设计的犰狳重复蛋白(dArmRPs)是模块化的,并且它们确实以模块化的方式结合细长的肽。他们的共有序列带有偏好精氨酸和赖氨酸的口袋。在为所有氨基酸侧链选择口袋的过程中,我们发现重复序列会导致在从文库中进行选择的过程中导致寄存器移位和肽翻转,从而阻碍了对新结合特异性的选择。为了解决这个问题,我们现在通过结合β-catenin的接枝,计算设计以及口袋和结合肽的相互优化来创建正交结合特异性。通过X射线结构确定,我们已经确认了设计和所需的相互作用。此外,我们可以通过单分子Förster共振能量转移确认溶液中不存在滑动。新的口袋可以从蛋白质的N端移到中间,保留其特性,从而进一步突出了系统的模块化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号