当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
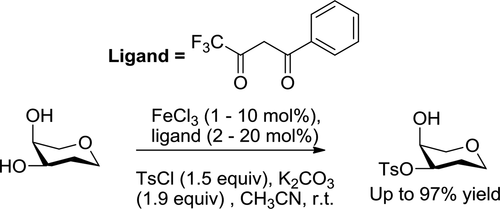
Regioselective Sulfonylation/Acylation of Carbohydrates Catalyzed by FeCl3 Combined with Benzoyltrifluoroacetone and Its Mechanism Study.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-01-27 , DOI: 10.1021/acs.joc.9b03128 Jian Lv 1 , Jia-Jia Zhu 1 , Yu Liu 1 , Hai Dong 1, 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-01-27 , DOI: 10.1021/acs.joc.9b03128 Jian Lv 1 , Jia-Jia Zhu 1 , Yu Liu 1 , Hai Dong 1, 2
Affiliation
|
A catalytic amount of FeCl3 combined with benzoyl trifluoroacetone (Hbtfa) (FeCl3/Hbtfa = 1/2) was used to catalyze sulfonylation/acylation of diols and polyols using diisopropylethylamine (DIPEA) or potassium carbonate (K2CO3) as a base. The catalytic system exhibited high catalytic activity, leading to excellent isolated yields of sulfonylation/acylation products with high regioselectivities. Mechanism studies indicated that FeCl3 initially formed [Fe(btfa)3] (btfa = benzoyl trifluoroacetonate) with twice the amount of Hbtfa under basic conditions in the solvent acetonitrile at room temperature. Then, Fe(btfa)3 and two hydroxyl groups of the substrates formed a five- or six-membered ring intermediate in the presence of the base. The subsequent reaction between the cyclic intermediate and a sulfonylation reagent led to the selective sulfonylation of the substrate. All key intermediates were captured in the high-resolution mass spectrometry assay, therefore demonstrating this mechanism for the first time.
中文翻译:

FeCl3结合苯甲酰三氟丙酮催化碳水化合物的区域选择性磺酰化/酰化反应及其机理研究。
使用二异丙基乙胺(DIPEA)或碳酸钾(K2CO3)作为碱,将催化量的FeCl3与苯甲酰基三氟丙酮(Hbtfa)(FeCl3 / Hbtfa = 1/2)结合用于催化二醇和多元醇的磺酰化/酰化。该催化体系表现出高催化活性,导致具有高区域选择性的磺酰化/酰化产物的优异分离产率。机理研究表明,FeCl3最初在碱性条件下于室温下于溶剂乙腈中以两倍的Hbtfa量形成[Fe(btfa)3](btfa =苯甲酰基三氟丙酮酸酯)。然后,在碱的存在下,Fe(btfa)3和底物的两个羟基形成五元或六元环中间体。环状中间体和磺酰化试剂之间的随后反应导致底物的选择性磺酰化。所有关键中间体均在高分辨率质谱分析中捕获,因此首次证明了这种机理。
更新日期:2020-01-27
中文翻译:

FeCl3结合苯甲酰三氟丙酮催化碳水化合物的区域选择性磺酰化/酰化反应及其机理研究。
使用二异丙基乙胺(DIPEA)或碳酸钾(K2CO3)作为碱,将催化量的FeCl3与苯甲酰基三氟丙酮(Hbtfa)(FeCl3 / Hbtfa = 1/2)结合用于催化二醇和多元醇的磺酰化/酰化。该催化体系表现出高催化活性,导致具有高区域选择性的磺酰化/酰化产物的优异分离产率。机理研究表明,FeCl3最初在碱性条件下于室温下于溶剂乙腈中以两倍的Hbtfa量形成[Fe(btfa)3](btfa =苯甲酰基三氟丙酮酸酯)。然后,在碱的存在下,Fe(btfa)3和底物的两个羟基形成五元或六元环中间体。环状中间体和磺酰化试剂之间的随后反应导致底物的选择性磺酰化。所有关键中间体均在高分辨率质谱分析中捕获,因此首次证明了这种机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号