当前位置:
X-MOL 学术
›
Sci. Total Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient oxidation and adsorption of As(III) and As(V) in water using a Fenton-like reagent, (ferrihydrite)-loaded biochar.
Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-01-27 , DOI: 10.1016/j.scitotenv.2020.136957 Yifan Huang 1 , Minling Gao 2 , Yingxuan Deng 3 , Zulqarnain Haider Khan 3 , Xuewei Liu 2 , Zhengguo Song 2 , Weiwen Qiu 4
Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-01-27 , DOI: 10.1016/j.scitotenv.2020.136957 Yifan Huang 1 , Minling Gao 2 , Yingxuan Deng 3 , Zulqarnain Haider Khan 3 , Xuewei Liu 2 , Zhengguo Song 2 , Weiwen Qiu 4
Affiliation
|
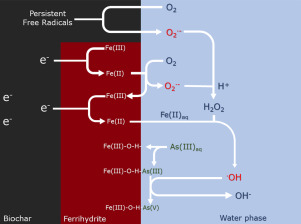
The by-product of the traditional Fenton reaction, colloidal arsenic-‑iron oxide, is migratable and may cause secondary environmental pollution. This paper reported a new strategy involving oxidizing and immobilizing inorganic arsenic using the Fenton reaction, and avoiding the risk of secondary contamination. Lab synthesized ferrihydrite-loaded biochar (FhBC) was developed for oxidizing and binding As(III) and As(V) in aqueous solution. Batch experiments and a series of spectrum analysis (e.g., X-ray photoelectron spectroscopy [XPS], electron paramagnetic resonance [EPR], and Fourier transform infrared spectroscopy [FTIR]) were conducted to study the oxidizing or adsorption capacity and mechanism. The maximum adsorption capacity of FhBC for As(III) and As(V) is 1.315 and 1.325 mmol/g, respectively. In addition, FhBC has an efficient oxidizing capacity within a wide pH range, which is because biochar promotes the Fenton reaction by acting as an electron donator, electron shuttler, or by providing persistent free radicals. Moreover, the adsorption mechanism was studied by FTIR spectroscopy, XPS, and X-ray diffraction (XRD). The formation of internal spherical complexes and iron oxides with a higher degree of crystallization was observed, which indicate that the products of adsorption are stable and robust in a complex environment and can exist in a highly crystallized form after adsorbing arsenic ions. Therefore, the use of FhBC as an adsorbent for arsenic represents a new strategy of using the Fenton reaction while reducing secondary contamination. These results may contribute to further mechanistic studies or extensive practical applications of FhBC.
中文翻译:

使用Fenton样试剂(三水铁矿)负载的生物炭有效地氧化和吸附水中的As(III)和As(V)。
传统芬顿反应的副产物胶体砷铁氧化物是可迁移的,可能造成二次环境污染。本文报道了一种新的策略,该策略涉及使用Fenton反应氧化和固定无机砷,并避免二次污染的风险。开发了实验室合成的负载三水铁矿的生物炭(FhBC),用于氧化和结合水溶液中的As(III)和As(V)。进行了批处理实验和一系列光谱分析(例如X射线光电子能谱[XPS],电子顺磁共振[EPR]和傅里叶变换红外光谱[FTIR]),以研究其氧化或吸附能力及其机理。FhBC对As(III)和As(V)的最大吸附容量分别为1.315和1.325 mmol / g。此外,FhBC在宽的pH范围内具有有效的氧化能力,这是因为生物炭通过充当电子给体,电子穿梭物或提供持久性自由基来促进Fenton反应。此外,通过FTIR光谱,XPS和X射线衍射(XRD)研究了吸附机理。观察到内部球形络合物和具有较高结晶度的氧化铁的形成,这表明在复杂的环境中,吸附产物是稳定且坚固的,并且在吸附砷离子后可以以高度结晶的形式存在。因此,使用FhBC作为砷的吸附剂代表了一种使用Fenton反应同时减少二次污染的新策略。这些结果可能有助于FhBC的进一步机理研究或广泛的实际应用。
更新日期:2020-01-27
中文翻译:

使用Fenton样试剂(三水铁矿)负载的生物炭有效地氧化和吸附水中的As(III)和As(V)。
传统芬顿反应的副产物胶体砷铁氧化物是可迁移的,可能造成二次环境污染。本文报道了一种新的策略,该策略涉及使用Fenton反应氧化和固定无机砷,并避免二次污染的风险。开发了实验室合成的负载三水铁矿的生物炭(FhBC),用于氧化和结合水溶液中的As(III)和As(V)。进行了批处理实验和一系列光谱分析(例如X射线光电子能谱[XPS],电子顺磁共振[EPR]和傅里叶变换红外光谱[FTIR]),以研究其氧化或吸附能力及其机理。FhBC对As(III)和As(V)的最大吸附容量分别为1.315和1.325 mmol / g。此外,FhBC在宽的pH范围内具有有效的氧化能力,这是因为生物炭通过充当电子给体,电子穿梭物或提供持久性自由基来促进Fenton反应。此外,通过FTIR光谱,XPS和X射线衍射(XRD)研究了吸附机理。观察到内部球形络合物和具有较高结晶度的氧化铁的形成,这表明在复杂的环境中,吸附产物是稳定且坚固的,并且在吸附砷离子后可以以高度结晶的形式存在。因此,使用FhBC作为砷的吸附剂代表了一种使用Fenton反应同时减少二次污染的新策略。这些结果可能有助于FhBC的进一步机理研究或广泛的实际应用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号