当前位置:
X-MOL 学术
›
J. Alloys Compd.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The adsorption property and mechanism for Hg(II) and Ag(I) by Schiff base functionalized magnetic Fe3O4 from aqueous solution
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.jallcom.2020.154051 Jianjian Zhao , Liping Luan , Ziwei Li , Zhaofeng Duan , Yufeng Li , Subin Zheng , Zhongxin Xue , Wenlong Xu , Yuzhong Niu
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.jallcom.2020.154051 Jianjian Zhao , Liping Luan , Ziwei Li , Zhaofeng Duan , Yufeng Li , Subin Zheng , Zhongxin Xue , Wenlong Xu , Yuzhong Niu
|
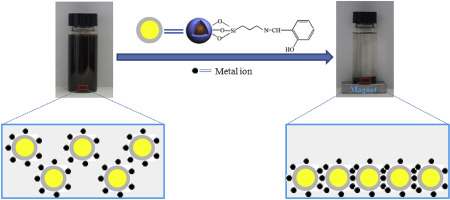
Abstract Schiff-base functionalized magnetic Fe3O4 were prepared by feasible one-pot subsequent homogeneous method (Fe3O4@SiO2–HO–S) and heterogeneous method (Fe3O4@SiO2-HE-S), respectively. The as-prepared adsorbents were used for the adsorption of aqueous Hg(II) and Ag(I). The effects of solution pH, time, temperature, coexisting metal ions, and initial metal ion concentration on the adsorption were investigated. The as-synthesized adsorbents were characterized by transmission electron microscope, scanning electron microscope, and X-ray photoelectron spectroscopy. The effect of solution pH indicates the optimal adsorption pH is 6. The adsorption equilibrium time are 180 and 220 min for both Hg(II) and Ag(I) under the concentration of 0.002 and 0.004 mol L−1, respectively. The adsorption kinetic process is proved to be dominated by film diffusion process and follows pseudo-second-order kinetic model. The adsorption isotherm process is proceeded by chemical mechanism and can be described by Langmuir model. Thermodynamic parameters indicate that the adsorption process is spontaneous, endothermic, and entropy increase. Adsorption selectivity demonstrates Fe3O4@SiO2-HE-S and Fe3O4@SiO2–HO–S exhibit good adsorption selectivity for Hg(II). The adsorption mechanism demonstrates the N atoms of Schiff base functional group mainly dominate the adsorption of Fe3O4@SiO2-HE-S and Fe3O4@SiO2–HO–S for metal ions. The regeneration property indicates the adsorbents can be reused with practical value.
中文翻译:

席夫碱功能化磁性Fe3O4从水溶液中吸附Hg(II)和Ag(I)的性质及机理
摘要 分别采用可行的一锅法均相法(Fe3O4@SiO2-HO-S)和异相法(Fe3O4@SiO2-HE-S)制备了席夫碱功能化磁性Fe3O4。所制备的吸附剂用于吸附含水 Hg(II) 和 Ag(I)。研究了溶液pH、时间、温度、共存金属离子和初始金属离子浓度对吸附的影响。合成的吸附剂通过透射电子显微镜、扫描电子显微镜和 X 射线光电子能谱进行表征。溶液pH的影响表明最佳吸附pH为6。在0.002和0.004 mol L-1的浓度下,Hg(II)和Ag(I)的吸附平衡时间分别为180和220分钟。证明吸附动力学过程以薄膜扩散过程为主,遵循准二级动力学模型。吸附等温线过程是通过化学机制进行的,可以用朗缪尔模型来描述。热力学参数表明吸附过程是自发的、吸热的、熵增加的。吸附选择性表明 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对 Hg(II) 表现出良好的吸附选择性。吸附机制表明席夫碱官能团的 N 原子主要支配 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对金属离子的吸附。再生性能表明吸附剂可以重复使用,具有实用价值。吸附等温线过程是通过化学机制进行的,可以用朗缪尔模型来描述。热力学参数表明吸附过程是自发的、吸热的、熵增加的。吸附选择性表明 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对 Hg(II) 表现出良好的吸附选择性。吸附机制表明席夫碱官能团的 N 原子主要支配 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对金属离子的吸附。再生性能表明吸附剂可以重复使用,具有实用价值。吸附等温线过程是通过化学机制进行的,可以用朗缪尔模型来描述。热力学参数表明吸附过程是自发的、吸热的、熵增加的。吸附选择性表明 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对 Hg(II) 表现出良好的吸附选择性。吸附机制表明席夫碱官能团的 N 原子主要支配 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对金属离子的吸附。再生性能表明吸附剂可以重复使用,具有实用价值。吸附机制表明席夫碱官能团的 N 原子主要支配 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对金属离子的吸附。再生性能表明吸附剂可以重复使用,具有实用价值。吸附机制表明席夫碱官能团的 N 原子主要支配 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对金属离子的吸附。再生性能表明吸附剂可以重复使用,具有实用价值。
更新日期:2020-06-01
中文翻译:

席夫碱功能化磁性Fe3O4从水溶液中吸附Hg(II)和Ag(I)的性质及机理
摘要 分别采用可行的一锅法均相法(Fe3O4@SiO2-HO-S)和异相法(Fe3O4@SiO2-HE-S)制备了席夫碱功能化磁性Fe3O4。所制备的吸附剂用于吸附含水 Hg(II) 和 Ag(I)。研究了溶液pH、时间、温度、共存金属离子和初始金属离子浓度对吸附的影响。合成的吸附剂通过透射电子显微镜、扫描电子显微镜和 X 射线光电子能谱进行表征。溶液pH的影响表明最佳吸附pH为6。在0.002和0.004 mol L-1的浓度下,Hg(II)和Ag(I)的吸附平衡时间分别为180和220分钟。证明吸附动力学过程以薄膜扩散过程为主,遵循准二级动力学模型。吸附等温线过程是通过化学机制进行的,可以用朗缪尔模型来描述。热力学参数表明吸附过程是自发的、吸热的、熵增加的。吸附选择性表明 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对 Hg(II) 表现出良好的吸附选择性。吸附机制表明席夫碱官能团的 N 原子主要支配 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对金属离子的吸附。再生性能表明吸附剂可以重复使用,具有实用价值。吸附等温线过程是通过化学机制进行的,可以用朗缪尔模型来描述。热力学参数表明吸附过程是自发的、吸热的、熵增加的。吸附选择性表明 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对 Hg(II) 表现出良好的吸附选择性。吸附机制表明席夫碱官能团的 N 原子主要支配 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对金属离子的吸附。再生性能表明吸附剂可以重复使用,具有实用价值。吸附等温线过程是通过化学机制进行的,可以用朗缪尔模型来描述。热力学参数表明吸附过程是自发的、吸热的、熵增加的。吸附选择性表明 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对 Hg(II) 表现出良好的吸附选择性。吸附机制表明席夫碱官能团的 N 原子主要支配 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对金属离子的吸附。再生性能表明吸附剂可以重复使用,具有实用价值。吸附机制表明席夫碱官能团的 N 原子主要支配 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对金属离子的吸附。再生性能表明吸附剂可以重复使用,具有实用价值。吸附机制表明席夫碱官能团的 N 原子主要支配 Fe3O4@SiO2-HE-S 和 Fe3O4@SiO2-HO-S 对金属离子的吸附。再生性能表明吸附剂可以重复使用,具有实用价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号