当前位置:
X-MOL 学术
›
J. Fluorine Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A de novo synthetic method to the access of N-substituted benzazepines
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-01-27 , DOI: 10.1016/j.jfluchem.2020.109466 Lamiaa Ouchakour , Melinda Nonn , Matthias D’hooghe , Loránd Kiss
中文翻译:

甲从头合成方法的接入N-取代的苯并吖庚因
更新日期:2020-01-27
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-01-27 , DOI: 10.1016/j.jfluchem.2020.109466 Lamiaa Ouchakour , Melinda Nonn , Matthias D’hooghe , Loránd Kiss
|
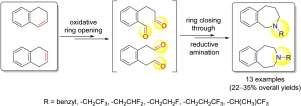
A novel, convenient procedure has been described for the construction of fluorine-containing benzazepines. The synthetic protocol starting from readily available dihydronaphthalene regioisomers is based on oxidative ring olefin bond cleavage followed by ring closure of the diformyl intermediates in the presence of some fluorine-containing primary amines across double reductive amination. The applicability of the developed synthetic method was demonstrated by the synthesis of 13 benzazepine compounds isolated in 22–35% overall yields.
中文翻译:

甲从头合成方法的接入N-取代的苯并吖庚因
已经描述了一种新颖,方便的方法用于构建含氟苯并ze庚因。从容易获得的二氢萘区域异构体开始的合成方案是基于氧化环烯烃键的裂解,然后在一些含氟伯胺的存在下通过双还原胺化对二甲酰基中间体进行闭环。合成的13种苯并ze庚因化合物的合成以22–35%的总收率证明了开发的合成方法的适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号