当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption of Cr(VI) on Al-substituted hematites and its reduction and retention in the presence of Fe2+ under conditions similar to subsurface soil environments.
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-01-27 , DOI: 10.1016/j.jhazmat.2019.122014 Shuqi Jiang 1 , Xinran Yan 2 , Caroline L Peacock 3 , Shuang Zhang 2 , Wei Li 2 , Jing Zhang 4 , Xionghan Feng 2 , Fan Liu 2 , Hui Yin 2
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-01-27 , DOI: 10.1016/j.jhazmat.2019.122014 Shuqi Jiang 1 , Xinran Yan 2 , Caroline L Peacock 3 , Shuang Zhang 2 , Wei Li 2 , Jing Zhang 4 , Xionghan Feng 2 , Fan Liu 2 , Hui Yin 2
Affiliation
|
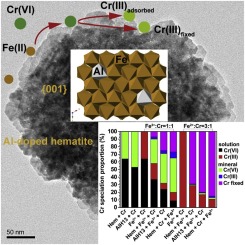
Aluminum substitution is common in iron (hydr)oxides in subsurface environments, and can significantly modify mineral interactions with contaminants. However, few studies investigate Cr(VI) adsorption and its subsequent mobility on Al-substituted iron (hydr)oxide surfaces. Here shows that Al substitution gradually modifies hematite crystals from {101}, {112}, {110} and {104} faceted rhombohedra to {001} faceted plates, resulting in a general decrease in Cr(VI) adsorption density and favoring of monodentate mononuclear over bidentate binuclear Cr(VI) adsorption complexes. Consequently, the mobility of Cr(VI) might be increased in environments with an abundance of Al-containing iron (hydr)oxides. However, pre-adsorption of Fe2+ on hematite promotes Cr(VI) adsorption, reduction and fixation, and Al-substituted hematite removes more Cr(VI) than pure hematite. Similarly, although addition of Fe2+ to Cr(VI)-adsorbed hematite remobilizes a small proportion of Cr, it greatly increases the proportion of Cr fixed. As the coexistence of Fe2+ and iron (hydr)oxides is common in subsurface environments, Al-containing iron (hydr)oxides will promote Cr(VI) uptake and retention, with a significant proportion fixed as Cr(III), limiting Cr mobility and toxicity. These results offer new insights into how iron (hydr)oxides might control the behaviors of other high-valence redox-sensitive contaminants, and provide a platform for modeling such processes in complex soil and sediment systems.
中文翻译:

在类似于地下土壤环境的条件下,Al-取代的赤铁矿上Cr(VI)的吸附及其在Fe2 +存在下的还原和保留。
铝替代在地下环境中的氧化铁中很常见,并且可以显着改变矿物与污染物的相互作用。但是,很少有研究调查Cr(VI)的吸附及其在Al取代的氧化铁(氢)氧化物表面上的迁移率。此处显示,Al取代逐渐将赤铁矿晶体从{101},{112},{110}和{104}切面菱形修改为{001}切面板,导致Cr(VI)吸附密度普遍降低,有利于单齿单齿双齿双核Cr(VI)吸附复合物。因此,在含有大量含铝的氧化铁的环境中,Cr(VI)的迁移率可能会增加。然而,铁离子在赤铁矿上的预吸附会促进Cr(VI)的吸附,还原和固定,铝取代的赤铁矿比纯赤铁矿去除更多的Cr(VI)。同样,尽管向吸附有Cr(VI)的赤铁矿中添加Fe2 +可以固定一小部分Cr,但它会大大增加固定的Cr的比例。由于Fe2 +和铁(氢)氧化物在地下环境中共存,含铝的铁(氢)氧化物将促进Cr(VI)的吸收和保留,其中很大一部分固定为Cr(III),限制了Cr的迁移和毒性。这些结果提供了新的见解,以了解氧化铁如何控制其他对高价氧化还原敏感的污染物的行为,并为在复杂的土壤和沉积物系统中模拟此类过程提供了平台。由于Fe2 +和铁(氢)氧化物在地下环境中共存,含铝的铁(氢)氧化物将促进Cr(VI)的吸收和保留,其中很大一部分固定为Cr(III),限制了Cr的迁移和毒性。这些结果提供了新的见解,以了解氧化铁如何控制其他对高价氧化还原敏感的污染物的行为,并为在复杂的土壤和沉积物系统中模拟此类过程提供了平台。由于Fe2 +和铁(氢)氧化物在地下环境中共存,含铝的铁(氢)氧化物将促进Cr(VI)的吸收和保留,其中很大一部分固定为Cr(III),限制了Cr的迁移和毒性。这些结果提供了新的见解,以了解氧化铁如何控制其他对高价氧化还原敏感的污染物的行为,并为在复杂的土壤和沉积物系统中模拟此类过程提供了平台。
更新日期:2020-01-27
中文翻译:

在类似于地下土壤环境的条件下,Al-取代的赤铁矿上Cr(VI)的吸附及其在Fe2 +存在下的还原和保留。
铝替代在地下环境中的氧化铁中很常见,并且可以显着改变矿物与污染物的相互作用。但是,很少有研究调查Cr(VI)的吸附及其在Al取代的氧化铁(氢)氧化物表面上的迁移率。此处显示,Al取代逐渐将赤铁矿晶体从{101},{112},{110}和{104}切面菱形修改为{001}切面板,导致Cr(VI)吸附密度普遍降低,有利于单齿单齿双齿双核Cr(VI)吸附复合物。因此,在含有大量含铝的氧化铁的环境中,Cr(VI)的迁移率可能会增加。然而,铁离子在赤铁矿上的预吸附会促进Cr(VI)的吸附,还原和固定,铝取代的赤铁矿比纯赤铁矿去除更多的Cr(VI)。同样,尽管向吸附有Cr(VI)的赤铁矿中添加Fe2 +可以固定一小部分Cr,但它会大大增加固定的Cr的比例。由于Fe2 +和铁(氢)氧化物在地下环境中共存,含铝的铁(氢)氧化物将促进Cr(VI)的吸收和保留,其中很大一部分固定为Cr(III),限制了Cr的迁移和毒性。这些结果提供了新的见解,以了解氧化铁如何控制其他对高价氧化还原敏感的污染物的行为,并为在复杂的土壤和沉积物系统中模拟此类过程提供了平台。由于Fe2 +和铁(氢)氧化物在地下环境中共存,含铝的铁(氢)氧化物将促进Cr(VI)的吸收和保留,其中很大一部分固定为Cr(III),限制了Cr的迁移和毒性。这些结果提供了新的见解,以了解氧化铁如何控制其他对高价氧化还原敏感的污染物的行为,并为在复杂的土壤和沉积物系统中模拟此类过程提供了平台。由于Fe2 +和铁(氢)氧化物在地下环境中共存,含铝的铁(氢)氧化物将促进Cr(VI)的吸收和保留,其中很大一部分固定为Cr(III),限制了Cr的迁移和毒性。这些结果提供了新的见解,以了解氧化铁如何控制其他对高价氧化还原敏感的污染物的行为,并为在复杂的土壤和沉积物系统中模拟此类过程提供了平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号