Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-01-27 , DOI: 10.1016/j.cej.2020.124230 Tianyuan Xu , Haodong Ji , Yu Gu , Tianyi Tong , Yabei Xia , Lizhi Zhang , Dongye Zhao
|
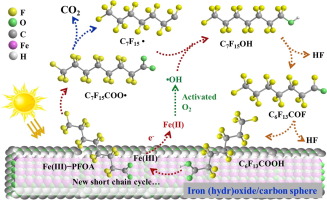
Perfluorooctanoic acid (PFOA) has been widely detected in aquatic systems. Yet, cost-effective technologies for degrading PFAS have been lacking. We prepared and tested an adsorptive photocatalyst consisting of iron (hydr)oxides and carbon spheres (FeO/CS) through a one-step hydrothermal process. Characterization results revealed that the presence of carbon spheres affected the crystal formation of iron (hydr)oxides, and resulted in mutually modified mixed phases ferrihydrite and carbon spheres. FeO/CS was able to effectively adsorb and then degrade the pre-sorbed PFOA under simulated solar light. FeO/CS(1:1), prepared at an Fe:Glucose molar ratio of 1:1, showed the highest PFOA adsorption capacity and photoactivity. At a dosage of 1.0 g/L, FeO/CS(1:1) adsorbed nearly all 200 µg/L of PFOA within 1 h, and when the PFOA-laden FeO/CS(1:1) was subjected to simulated solar light at neutral pH, 95.2% of pre-concentrated PFOA was photodegraded and 57.2% defluorinated in 4 h. The efficient degradation also regenerated the material, allowing for repeated uses of the material without chemical regeneration. The much enhanced adsorption and photocatalytic activity of FeO/CS was attributed to: 1) CS facilitated formation of ferrihydrite, leading to adsorption of PFOA via binuclear and bidentate complexation, and 2) a hybrid ferrihydrite-CS structure, enabling multi-point, corporative adsorption of PFOA, and increasing direct electron extraction from PFOA under solar light irradiation. Moreover, •OH radicals played an important role in PFOA degradation. Lastly, a photodegradation mechanism is proposed based on experimental findings and density functional theory calculations.
中文翻译:

氧化铁/碳球复合物增强水中全氟辛酸的吸附和光催化降解
全氟辛酸(PFOA)已在水生系统中被广泛检测到。但是,缺乏用于降解PFAS的具有成本效益的技术。我们通过一步水热法制备并测试了由铁(氢)氧化物和碳球(FeO / CS)组成的吸附性光催化剂。表征结果表明,碳球的存在影响了铁(氢)氧化物的晶体形成,并导致了互变混合相的水铁矿和碳球。FeO / CS能够在模拟太阳光下有效吸附然后降解预吸附的PFOA。以Fe:葡萄糖摩尔比为1:1制备的FeO / CS(1:1)具有最高的PFOA吸附能力和光活性。当剂量为1.0 g / L时,FeO / CS(1:1)在1 h内以及负载PFOA的FeO / CS(1:1)在中性pH下接受模拟太阳光照射,在4小时内95.2%的预浓缩PFOA被光降解,而57.2%的被脱氟。有效降解还使材料再生,从而无需化学再生即可重复使用该材料。FeO / CS的吸附和光催化活性大大增强,其原因是:1)CS促进了水铁矿的形成,从而通过双核和双齿络合作用吸附了PFOA; 2)杂化的铁水铁矿-CS结构,可实现多点,多价吸收PFOA,并在阳光照射下增加从PFOA的直接电子提取。此外,允许重复使用该材料而无需化学再生。FeO / CS的吸附和光催化活性大大增强,其原因是:1)CS促进了水铁矿的形成,从而通过双核和双齿络合作用吸附了PFOA; 2)杂化的铁水铁矿-CS结构,可实现多点,多价吸收PFOA,并增加在太阳光照射下从PFOA直接提取电子的能力。此外,允许重复使用该材料而无需化学再生。FeO / CS的吸附和光催化活性大大增强,其原因是:1)CS促进了水铁矿的形成,从而通过双核和双齿络合作用吸附了PFOA,以及2)杂化的铁水铁矿-CS结构,使得多点,可吸收PFOA,并增加在太阳光照射下从PFOA直接提取电子的能力。此外,并在日光照射下增加了从PFOA中直接提取电子的能力。此外,并在日光照射下增加了从PFOA中直接提取电子的能力。此外,• OH自由基在PFOA降解中起重要作用。最后,基于实验结果和密度泛函理论计算,提出了一种光降解机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号