当前位置:
X-MOL 学术
›
Mol. Psychiatry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Success and efficiency of phase 2/3 adjunctive trials for MDD funded by industry: a systematic review.
Molecular Psychiatry ( IF 9.6 ) Pub Date : 2020-01-27 , DOI: 10.1038/s41380-020-0646-3 Naji C Salloum 1 , Maurizio Fava 1 , Sophia Ball 1 , George I Papakostas 1
Molecular Psychiatry ( IF 9.6 ) Pub Date : 2020-01-27 , DOI: 10.1038/s41380-020-0646-3 Naji C Salloum 1 , Maurizio Fava 1 , Sophia Ball 1 , George I Papakostas 1
Affiliation
|
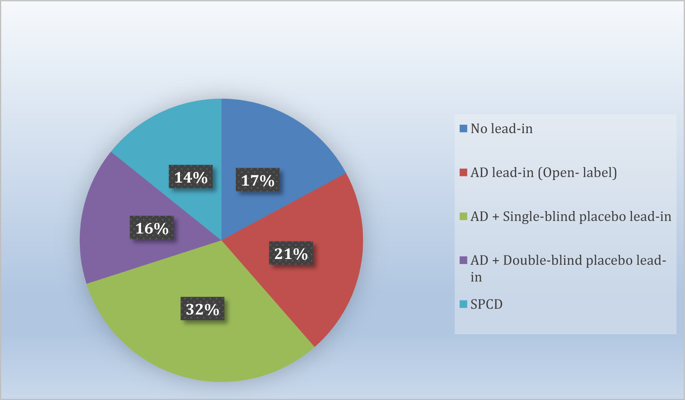
To review the success rate and efficiency of industry-sponsored phase 2/3 clinical trials for adjunctive therapies for antidepressant partial- and non-responders with major depressive disorder (MDD), a systematic search of Pubmed/Medline was conducted, in addition to abstracts of major psychiatric meeting held since 2010, of randomized, placebo-controlled adjunct oral pharmacotherapy trials in this patient population. Forty-six (n = 33,900; 70 drug compactor arms) trials were pooled, yielding only three approved drugs. Twenty-two (31.4%) drug-placebo comparisons were successful. Numerically, success rates for treatment arms from studies with one versus more than one drug-placebo comparison were higher (39.3% versus 26.2%). The antidepressant lead-in employing single-blind placebo and the sequential-parallel comparison design (SPCD) were successful in 50% and 40% of cases, respectively. The direct randomization (no lead-in) design yielded positive results in one third of cases. The success rate of open-label antidepressant lead-ins without placebo or using double-blind placebo was very poor (<15%). There was also a pronounced discrepancy in terms of efficiency across study designs. Accounting for sample size requirements, a phase 3 program using SPCD would have a higher cumulative chance of yielding two positive trials (50%) than a phase 3 program using a single-blind placebo lead-in (40%). Future programs should carefully weigh the need for a lead-in, which is time-consuming, expensive and, in some cases (i.e., open-label antidepressant without placebo or with double-blind placebo) nearly futile. Instead, more effort should involve the use of studies where patients are directly randomized, such as the SPCD, with more investment shifted towards the accurate and independent vetting of subject eligibility.
中文翻译:

由工业界资助的MDD第2/3期辅助试验的成功与效率:系统评价。
为了回顾行业赞助的2/3期重度抑郁症(MDD)抗抑郁药部分和非反应性辅助治疗的辅助疗法的成功率和效率,除了摘要之外,还进行了系统的Pubmed / Medline搜索自2010年以来举行的主要精神病学会议中,针对该患者人群进行了随机,安慰剂对照的辅助口服药物治疗试验。汇总了四十六项(n = 33,900; 70个压实机臂)试验,仅产生了三种批准的药物。22(31.4%)药物-安慰剂比较成功。从数值上讲,一项与一项以上的药物-安慰剂比较研究的治疗组成功率更高(39.3%对26.2%)。采用单盲安慰剂和顺序平行比较设计(SPCD)的抗抑郁药分别成功治疗了50%和40%的病例。直接随机化(无导入)设计在三分之一的情况下产生了积极的结果。不使用安慰剂或使用双盲安慰剂的抗标签抗抑郁药物的成功率非常低(<15%)。整个研究设计在效率方面也存在明显差异。考虑到样本量的要求,使用SPCD的第3阶段计划比使用单盲安慰剂导入的第3阶段计划(40%)具有更高的产生两次阳性试验(50%)的累积机会。未来的计划应仔细权衡导入的需求,这是耗时,昂贵且在某些情况下(例如,不含安慰剂或含双盲安慰剂的开放标签抗抑郁药几乎无效。取而代之的是,应该进行更多的努力,如直接将患者随机分组的研究,例如SPCD,并将更多的投资转向对受试者资格的准确和独立的审查。
更新日期:2020-01-27
中文翻译:

由工业界资助的MDD第2/3期辅助试验的成功与效率:系统评价。
为了回顾行业赞助的2/3期重度抑郁症(MDD)抗抑郁药部分和非反应性辅助治疗的辅助疗法的成功率和效率,除了摘要之外,还进行了系统的Pubmed / Medline搜索自2010年以来举行的主要精神病学会议中,针对该患者人群进行了随机,安慰剂对照的辅助口服药物治疗试验。汇总了四十六项(n = 33,900; 70个压实机臂)试验,仅产生了三种批准的药物。22(31.4%)药物-安慰剂比较成功。从数值上讲,一项与一项以上的药物-安慰剂比较研究的治疗组成功率更高(39.3%对26.2%)。采用单盲安慰剂和顺序平行比较设计(SPCD)的抗抑郁药分别成功治疗了50%和40%的病例。直接随机化(无导入)设计在三分之一的情况下产生了积极的结果。不使用安慰剂或使用双盲安慰剂的抗标签抗抑郁药物的成功率非常低(<15%)。整个研究设计在效率方面也存在明显差异。考虑到样本量的要求,使用SPCD的第3阶段计划比使用单盲安慰剂导入的第3阶段计划(40%)具有更高的产生两次阳性试验(50%)的累积机会。未来的计划应仔细权衡导入的需求,这是耗时,昂贵且在某些情况下(例如,不含安慰剂或含双盲安慰剂的开放标签抗抑郁药几乎无效。取而代之的是,应该进行更多的努力,如直接将患者随机分组的研究,例如SPCD,并将更多的投资转向对受试者资格的准确和独立的审查。











































 京公网安备 11010802027423号
京公网安备 11010802027423号