Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Augmenting Tumor‐Starvation Therapy by Cancer Cell Autophagy Inhibition
Advanced Science ( IF 14.3 ) Pub Date : 2020-01-27 , DOI: 10.1002/advs.201902847 Bowen Yang 1, 2 , Li Ding 1 , Yu Chen 1, 2 , Jianlin Shi 1, 2
Advanced Science ( IF 14.3 ) Pub Date : 2020-01-27 , DOI: 10.1002/advs.201902847 Bowen Yang 1, 2 , Li Ding 1 , Yu Chen 1, 2 , Jianlin Shi 1, 2
Affiliation
|
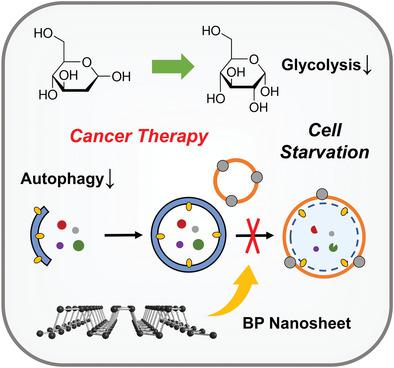
It was recently recognized that cancer therapeutic efficacy may be greatly compromised by an intrinsic protective mechanism called autophagy, by which cancer cells survive in harsh conditions such as starvation. Here, a synergetic strategy is described for cancer treatment by suppressing such a protective mechanism for augmenting tumor‐starvation therapy. The synergetic therapy is achieved by restraining glucose metabolism using an antiglycolytic agent to predispose cancer cells to severe energy deprivation; concurrently the downstream autophagic flux and compensatory energy supplies are blocked by the autophagy inhibitor black phosphorus nanosheet. Cancer cells fail to extract their own nutrient to feed themselves, finally succumbing to therapeutic interventions and starving to death. Both in vitro and in vivo results evidence the cooperative effect between the autophagy inhibitor and antiglycolytic agent, which leads to remarkable synergetic antineoplastic outcome. It is expected that such a combinational approach by concurrently blocking exogenous and endogenous nutrition supplies will be beneficial to the design of effective tumor‐specific cancer therapies in the future.
中文翻译:

通过抑制癌细胞自噬增强肿瘤饥饿疗法
最近人们认识到,癌症治疗效果可能会受到一种称为自噬的内在保护机制的极大影响,通过这种机制,癌细胞可以在饥饿等恶劣条件下生存。在这里,描述了一种通过抑制这种增强肿瘤饥饿疗法的保护机制来治疗癌症的协同策略。协同疗法是通过使用抗糖酵解剂抑制葡萄糖代谢来实现的,使癌细胞容易严重缺乏能量。同时,下游自噬通量和补偿性能量供应被自噬抑制剂黑磷纳米片阻断。癌细胞无法提取自己的营养来养活自己,最终屈服于治疗干预并饿死。体外和体内结果都证明了自噬抑制剂和抗糖酵解药物之间的协同作用,从而产生显着的协同抗肿瘤效果。预计这种同时阻断外源性和内源性营养供应的组合方法将有利于未来有效的肿瘤特异性癌症疗法的设计。
更新日期:2020-01-27
中文翻译:

通过抑制癌细胞自噬增强肿瘤饥饿疗法
最近人们认识到,癌症治疗效果可能会受到一种称为自噬的内在保护机制的极大影响,通过这种机制,癌细胞可以在饥饿等恶劣条件下生存。在这里,描述了一种通过抑制这种增强肿瘤饥饿疗法的保护机制来治疗癌症的协同策略。协同疗法是通过使用抗糖酵解剂抑制葡萄糖代谢来实现的,使癌细胞容易严重缺乏能量。同时,下游自噬通量和补偿性能量供应被自噬抑制剂黑磷纳米片阻断。癌细胞无法提取自己的营养来养活自己,最终屈服于治疗干预并饿死。体外和体内结果都证明了自噬抑制剂和抗糖酵解药物之间的协同作用,从而产生显着的协同抗肿瘤效果。预计这种同时阻断外源性和内源性营养供应的组合方法将有利于未来有效的肿瘤特异性癌症疗法的设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号