当前位置:
X-MOL 学术
›
Nat. Nanotechnol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Real-time mass spectrometric characterization of the solid-electrolyte interphase of a lithium-ion battery.
Nature Nanotechnology ( IF 38.1 ) Pub Date : 2020-01-27 , DOI: 10.1038/s41565-019-0618-4 Yufan Zhou 1, 2 , Mao Su 3, 4, 5 , Xiaofei Yu 1, 2 , Yanyan Zhang 1 , Jun-Gang Wang 1 , Xiaodi Ren 6 , Ruiguo Cao 6 , Wu Xu 6 , Donald R Baer 1 , Yingge Du 3 , Oleg Borodin 7, 8 , Yanting Wang 4, 5 , Xue-Lin Wang 2 , Kang Xu 7, 8 , Zhijie Xu 3 , Chongmin Wang 1 , Zihua Zhu 1
Nature Nanotechnology ( IF 38.1 ) Pub Date : 2020-01-27 , DOI: 10.1038/s41565-019-0618-4 Yufan Zhou 1, 2 , Mao Su 3, 4, 5 , Xiaofei Yu 1, 2 , Yanyan Zhang 1 , Jun-Gang Wang 1 , Xiaodi Ren 6 , Ruiguo Cao 6 , Wu Xu 6 , Donald R Baer 1 , Yingge Du 3 , Oleg Borodin 7, 8 , Yanting Wang 4, 5 , Xue-Lin Wang 2 , Kang Xu 7, 8 , Zhijie Xu 3 , Chongmin Wang 1 , Zihua Zhu 1
Affiliation
|
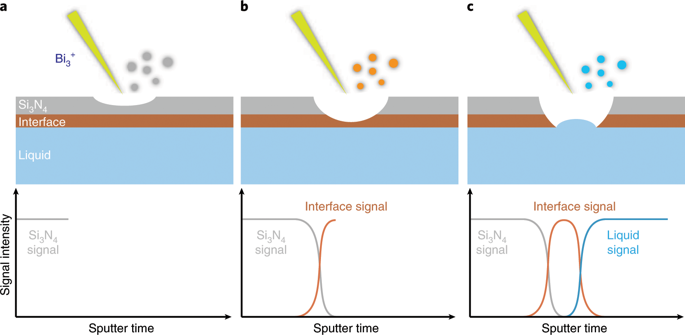
The solid-electrolyte interphase (SEI) dictates the performance of most batteries, but the understanding of its chemistry and structure is limited by the lack of in situ experimental tools. In this work, we present a dynamic picture of the SEI formation in lithium-ion batteries using in operando liquid secondary ion mass spectrometry in combination with molecular dynamics simulations. We find that before any interphasial chemistry occurs (during the initial charging), an electric double layer forms at the electrode/electrolyte interface due to the self-assembly of solvent molecules. The formation of the double layer is directed by Li+ and the electrode surface potential. The structure of this double layer predicts the eventual interphasial chemistry; in particular, the negatively charged electrode surface repels salt anions from the inner layer and results in an inner SEI that is thin, dense and inorganic in nature. It is this dense layer that is responsible for conducting Li+ and insulating electrons, the main functions of the SEI. An electrolyte-permeable and organic-rich outer layer appears after the formation of the inner layer. In the presence of a highly concentrated, fluoride-rich electrolyte, the inner SEI layer has an elevated concentration of LiF due to the presence of anions in the double layer. These real-time nanoscale observations will be helpful in engineering better interphases for future batteries.
中文翻译:

锂离子电池固体电解质中间相的实时质谱表征。
固体电解质中间相(SEI)决定了大多数电池的性能,但是由于缺乏原位实验工具,对化学和结构的了解受到限制。在这项工作中,我们提供了动态锂离子电池SEI形成的动态图,该锂离子电池在操作中使用了液体二次离子质谱,并结合了分子动力学模拟。我们发现在任何相间化学发生之前(在初始充电期间),由于溶剂分子的自组装,在电极/电解质界面形成了双电层。双层的形成由Li +和电极表面电势指导。这个双层的结构预示着最终的相间化学。特别是,带负电的电极表面会从内层排斥盐阴离子,从而导致内部的SEI本质上稀薄,致密和无机。正是这种致密层负责传导Li +和绝缘电子,这是SEI的主要功能。在形成内层之后出现电解质可渗透且富含有机物的外层。在高浓度,富含氟化物的电解质的存在下,由于双层中存在阴离子,内部SEI层的LiF浓度升高。这些实时的纳米级观测将有助于为将来的电池设计更好的中间相。在形成内层之后出现电解质可渗透且富含有机物的外层。在高浓度,富含氟化物的电解质的存在下,由于双层中存在阴离子,内部SEI层的LiF浓度升高。这些实时的纳米级观测将有助于为将来的电池设计更好的中间相。在形成内层之后出现电解质可渗透且富含有机物的外层。在高浓度,富含氟化物的电解质的存在下,由于双层中存在阴离子,内部SEI层的LiF浓度升高。这些实时的纳米级观测将有助于为将来的电池设计更好的中间相。
更新日期:2020-01-27
中文翻译:

锂离子电池固体电解质中间相的实时质谱表征。
固体电解质中间相(SEI)决定了大多数电池的性能,但是由于缺乏原位实验工具,对化学和结构的了解受到限制。在这项工作中,我们提供了动态锂离子电池SEI形成的动态图,该锂离子电池在操作中使用了液体二次离子质谱,并结合了分子动力学模拟。我们发现在任何相间化学发生之前(在初始充电期间),由于溶剂分子的自组装,在电极/电解质界面形成了双电层。双层的形成由Li +和电极表面电势指导。这个双层的结构预示着最终的相间化学。特别是,带负电的电极表面会从内层排斥盐阴离子,从而导致内部的SEI本质上稀薄,致密和无机。正是这种致密层负责传导Li +和绝缘电子,这是SEI的主要功能。在形成内层之后出现电解质可渗透且富含有机物的外层。在高浓度,富含氟化物的电解质的存在下,由于双层中存在阴离子,内部SEI层的LiF浓度升高。这些实时的纳米级观测将有助于为将来的电池设计更好的中间相。在形成内层之后出现电解质可渗透且富含有机物的外层。在高浓度,富含氟化物的电解质的存在下,由于双层中存在阴离子,内部SEI层的LiF浓度升高。这些实时的纳米级观测将有助于为将来的电池设计更好的中间相。在形成内层之后出现电解质可渗透且富含有机物的外层。在高浓度,富含氟化物的电解质的存在下,由于双层中存在阴离子,内部SEI层的LiF浓度升高。这些实时的纳米级观测将有助于为将来的电池设计更好的中间相。










































 京公网安备 11010802027423号
京公网安备 11010802027423号