Nature Medicine ( IF 58.7 ) Pub Date : 2020-01-27 , DOI: 10.1038/s41591-019-0735-5 Donald B Kohn 1 , Claire Booth 2 , Elizabeth M Kang 3 , Sung-Yun Pai 4 , Kit L Shaw 1 , Giorgia Santilli 2 , Myriam Armant 4 , Karen F Buckland 2 , Uimook Choi 3 , Suk See De Ravin 3 , Morna J Dorsey 5 , Caroline Y Kuo 1 , Diego Leon-Rico 2 , Christine Rivat 2 , Natalia Izotova 2 , Kimberly Gilmour 2 , Katie Snell 2 , Jinhua Xu-Bayford Dip 2 , Jinan Darwish 2 , Emma C Morris 6 , Dayna Terrazas 1 , Leo D Wang 4, 7 , Christopher A Bauser 8 , Tobias Paprotka 8 , Douglas B Kuhns 9 , John Gregg 10 , Hayley E Raymond 10 , John K Everett 10 , Geraldine Honnet 11 , Luca Biasco 2 , Peter E Newburger 12 , Frederic D Bushman 10 , Manuel Grez 13 , H Bobby Gaspar 2, 14 , David A Williams 4 , Harry L Malech 3 , Anne Galy 11, 15 , Adrian J Thrasher 2 ,
|
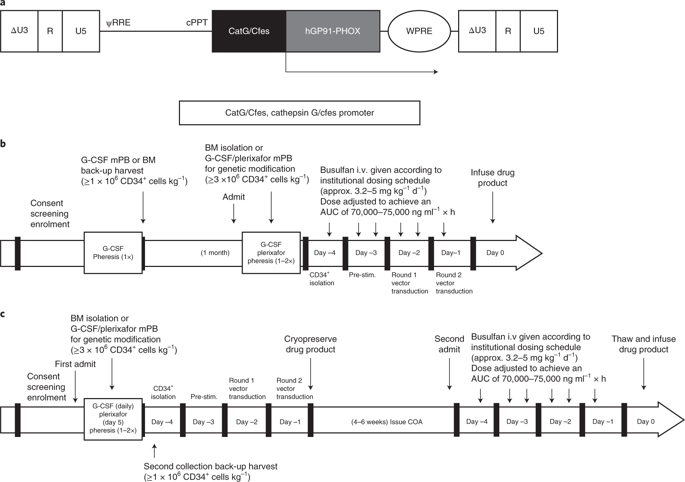
Chronic granulomatous disease (CGD) is a rare inherited disorder of phagocytic cells1,2. We report the initial results of nine severely affected X-linked CGD (X-CGD) patients who received ex vivo autologous CD34+ hematopoietic stem and progenitor cell-based lentiviral gene therapy following myeloablative conditioning in first-in-human studies (trial registry nos. NCT02234934 and NCT01855685). The primary objectives were to assess the safety and evaluate the efficacy and stability of biochemical and functional reconstitution in the progeny of engrafted cells at 12 months. The secondary objectives included the evaluation of augmented immunity against bacterial and fungal infection, as well as assessment of hematopoietic stem cell transduction and engraftment. Two enrolled patients died within 3 months of treatment from pre-existing comorbidities. At 12 months, six of the seven surviving patients demonstrated stable vector copy numbers (0.4–1.8 copies per neutrophil) and the persistence of 16–46% oxidase-positive neutrophils. There was no molecular evidence of either clonal dysregulation or transgene silencing. Surviving patients have had no new CGD-related infections, and six have been able to discontinue CGD-related antibiotic prophylaxis. The primary objective was met in six of the nine patients at 12 months follow-up, suggesting that autologous gene therapy is a promising approach for CGD patients.
中文翻译:

X连锁慢性肉芽肿病的慢病毒基因治疗
慢性肉芽肿病 (CGD) 是一种罕见的吞噬细胞遗传性疾病1,2 。我们报告了 9 名严重受影响的 X 连锁 CGD (X-CGD) 患者的初步结果,这些患者在首次人体研究中进行清髓性调理后接受离体自体 CD34 +造血干细胞和祖细胞慢病毒基因治疗(试验注册号.NCT02234934 和 NCT01855685)。主要目的是评估移植细胞后代在 12 个月时的安全性以及生化和功能重建的有效性和稳定性。次要目标包括评估针对细菌和真菌感染的增强免疫力,以及评估造血干细胞转导和植入。两名入组患者在治疗后 3 个月内因先前存在的合并症死亡。 12 个月时,7 名幸存患者中的 6 名表现出稳定的载体拷贝数(每个中性粒细胞 0.4-1.8 拷贝)和 16-46% 氧化酶阳性中性粒细胞的持续存在。没有克隆失调或转基因沉默的分子证据。幸存患者没有出现新的 CGD 相关感染,其中 6 名患者已经能够停止 CGD 相关抗生素预防。在 12 个月的随访中,9 名患者中有 6 名达到了主要目标,这表明自体基因治疗对于 CGD 患者来说是一种有前途的方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号