Nature Medicine ( IF 58.7 ) Pub Date : 2020-01-27 , DOI: 10.1038/s41591-019-0738-2 A Moretti 1 , L Fonteyne 2 , F Giesert 3 , P Hoppmann 1 , A B Meier 1 , T Bozoglu 1 , A Baehr 1 , C M Schneider 1 , D Sinnecker 1 , K Klett 1 , T Fröhlich 2, 4 , F Abdel Rahman 1 , T Haufe 1 , S Sun 1 , V Jurisch 1 , B Kessler 2, 4 , R Hinkel 1 , R Dirschinger 1 , E Martens 1 , C Jilek 1 , A Graf 2, 4 , S Krebs 2, 4 , G Santamaria 1 , M Kurome 2, 4 , V Zakhartchenko 2, 4 , B Campbell 1 , K Voelse 5 , A Wolf 1 , T Ziegler 1 , S Reichert 6 , S Lee 1 , F Flenkenthaler 2, 4 , T Dorn 1 , I Jeremias 5 , H Blum 2, 4 , A Dendorfer 7 , A Schnieke 8 , S Krause 6 , M C Walter 6 , N Klymiuk 2, 4 , K L Laugwitz 1 , E Wolf 2, 4 , W Wurst 3, 9 , C Kupatt 1
|
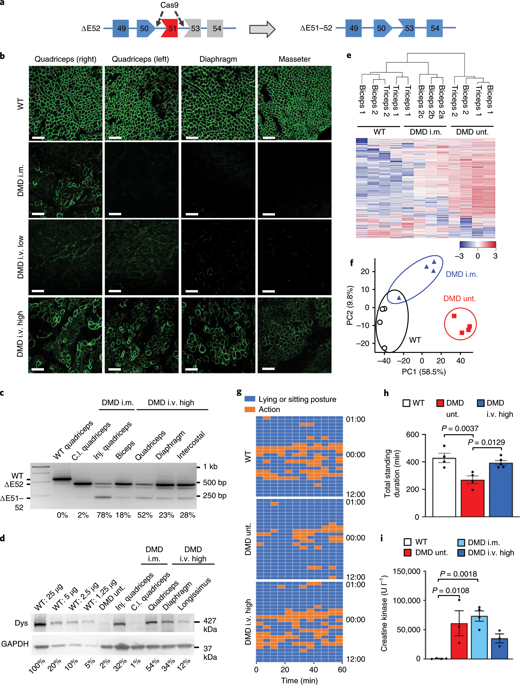
Frameshift mutations in the DMD gene, encoding dystrophin, cause Duchenne muscular dystrophy (DMD), leading to terminal muscle and heart failure in patients. Somatic gene editing by sequence-specific nucleases offers new options for restoring the DMD reading frame, resulting in expression of a shortened but largely functional dystrophin protein. Here, we validated this approach in a pig model of DMD lacking exon 52 of DMD (DMDΔ52), as well as in a corresponding patient-derived induced pluripotent stem cell model. In DMDΔ52 pigs1, intramuscular injection of adeno-associated viral vectors of serotype 9 carrying an intein-split Cas9 (ref. 2) and a pair of guide RNAs targeting sequences flanking exon 51 (AAV9-Cas9-gE51) induced expression of a shortened dystrophin (DMDΔ51–52) and improved skeletal muscle function. Moreover, systemic application of AAV9-Cas9-gE51 led to widespread dystrophin expression in muscle, including diaphragm and heart, prolonging survival and reducing arrhythmogenic vulnerability. Similarly, in induced pluripotent stem cell-derived myoblasts and cardiomyocytes of a patient lacking DMDΔ52, AAV6-Cas9-g51-mediated excision of exon 51 restored dystrophin expression and amelioreate skeletal myotube formation as well as abnormal cardiomyocyte Ca2+ handling and arrhythmogenic susceptibility. The ability of Cas9-mediated exon excision to improve DMD pathology in these translational models paves the way for new treatment approaches in patients with this devastating disease.
中文翻译:

体细胞基因编辑可改善猪和人类杜氏肌营养不良模型的骨骼和心肌衰竭
编码抗肌营养不良蛋白的DMD基因中的移码突变会导致杜氏肌营养不良症 (DMD),从而导致患者出现终末肌肉和心力衰竭。通过序列特异性核酸酶进行的体细胞基因编辑为恢复DMD阅读框提供了新的选择,从而表达缩短但主要具有功能的肌营养不良蛋白。在这里,我们在缺乏DMD外显子 52 ( DMD Δ52) 的 DMD 猪模型以及相应的患者来源的诱导多能干细胞模型中验证了这种方法。在DMD Δ52 猪1中,肌肉注射血清型 9 的腺相关病毒载体,该载体携带内含肽分裂 Cas9(参考文献2 )和一对位于外显子 51 侧翼的引导 RNA 靶向序列(AAV9-Cas9-gE51),诱导表达缩短肌营养不良蛋白 (DMDΔ51–52) 并改善骨骼肌功能。此外,AAV9-Cas9-gE51的全身应用导致肌营养不良蛋白在肌肉(包括膈肌和心脏)中广泛表达,从而延长了生存期并减少了致心律失常的脆弱性。同样,在缺乏DMDΔ52的患者的诱导多能干细胞来源的成肌细胞和心肌细胞中,AAV6-Cas9-g51 介导的外显子 51 切除恢复了抗肌营养不良蛋白表达并改善骨骼肌管形成以及异常心肌细胞 Ca 2+处理和致心律失常易感性。 Cas9介导的外显子切除能够改善这些转化模型中的DMD病理学,为患有这种毁灭性疾病的患者的新治疗方法铺平了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号