当前位置:
X-MOL 学术
›
Nat. Biomed. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips.
Nature Biomedical Engineering ( IF 26.8 ) Pub Date : 2020-01-27 , DOI: 10.1038/s41551-019-0498-9 Anna Herland 1, 2, 3 , Ben M Maoz 1, 4, 5, 6 , Debarun Das 7 , Mahadevabharath R Somayaji 7 , Rachelle Prantil-Baun 1 , Richard Novak 1 , Michael Cronce 1 , Tessa Huffstater 1 , Sauveur S F Jeanty 1 , Miles Ingram 1 , Angeliki Chalkiadaki 1 , David Benson Chou 1 , Susan Marquez 1 , Aaron Delahanty 1 , Sasan Jalili-Firoozinezhad 1, 8 , Yuka Milton 1 , Alexandra Sontheimer-Phelps 1, 9 , Ben Swenor 1 , Oren Levy 1 , Kevin K Parker 1, 4 , Andrzej Przekwas 7 , Donald E Ingber 1, 2, 10
Nature Biomedical Engineering ( IF 26.8 ) Pub Date : 2020-01-27 , DOI: 10.1038/s41551-019-0498-9 Anna Herland 1, 2, 3 , Ben M Maoz 1, 4, 5, 6 , Debarun Das 7 , Mahadevabharath R Somayaji 7 , Rachelle Prantil-Baun 1 , Richard Novak 1 , Michael Cronce 1 , Tessa Huffstater 1 , Sauveur S F Jeanty 1 , Miles Ingram 1 , Angeliki Chalkiadaki 1 , David Benson Chou 1 , Susan Marquez 1 , Aaron Delahanty 1 , Sasan Jalili-Firoozinezhad 1, 8 , Yuka Milton 1 , Alexandra Sontheimer-Phelps 1, 9 , Ben Swenor 1 , Oren Levy 1 , Kevin K Parker 1, 4 , Andrzej Przekwas 7 , Donald E Ingber 1, 2, 10
Affiliation
|
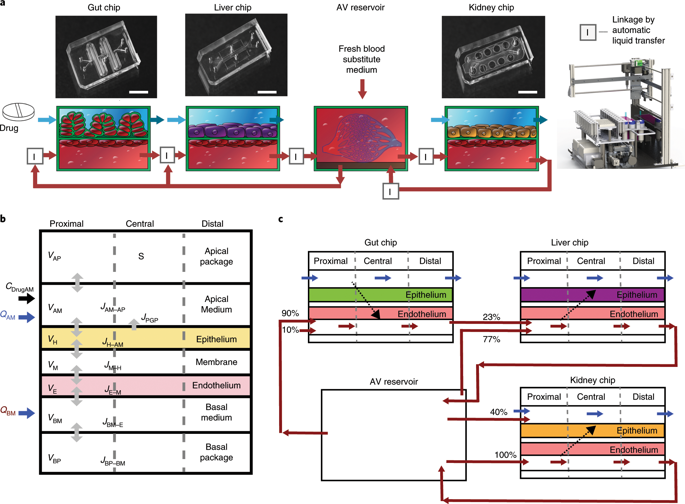
Analyses of drug pharmacokinetics (PKs) and pharmacodynamics (PDs) performed in animals are often not predictive of drug PKs and PDs in humans, and in vitro PK and PD modelling does not provide quantitative PK parameters. Here, we show that physiological PK modelling of first-pass drug absorption, metabolism and excretion in humans-using computationally scaled data from multiple fluidically linked two-channel organ chips-predicts PK parameters for orally administered nicotine (using gut, liver and kidney chips) and for intravenously injected cisplatin (using coupled bone marrow, liver and kidney chips). The chips are linked through sequential robotic liquid transfers of a common blood substitute by their endothelium-lined channels (as reported by Novak et al. in an associated Article) and share an arteriovenous fluid-mixing reservoir. We also show that predictions of cisplatin PDs match previously reported patient data. The quantitative in-vitro-to-in-vivo translation of PK and PD parameters and the prediction of drug absorption, distribution, metabolism, excretion and toxicity through fluidically coupled organ chips may improve the design of drug-administration regimens for phase-I clinical trials.
中文翻译:

通过流体耦合血管化器官芯片定量预测人体对药物的药代动力学反应。
在动物中进行的药物药代动力学 (PK) 和药效学 (PD) 分析通常不能预测人体中的药物 PK 和 PD,并且体外 PK 和 PD 模型不提供定量 PK 参数。在这里,我们展示了人体首过药物吸收、代谢和排泄的生理 PK 模型(使用来自多个流体连接的双通道器官芯片的计算缩放数据)预测口服尼古丁的 PK 参数(使用肠道、肝脏和肾脏芯片) )和静脉注射顺铂(使用偶联的骨髓、肝脏和肾脏芯片)。这些芯片通过其内皮通道(如 Novak 等人在相关文章中报道的那样)通过常见血液替代品的顺序机器人液体传输进行连接,并共享动静脉流体混合储液器。我们还表明,顺铂 PD 的预测与之前报告的患者数据相符。通过流体耦合器官芯片定量体外到体内PK和PD参数的转换以及药物吸收、分布、代谢、排泄和毒性的预测可以改善I期临床给药方案的设计试验。
更新日期:2020-01-27
中文翻译:

通过流体耦合血管化器官芯片定量预测人体对药物的药代动力学反应。
在动物中进行的药物药代动力学 (PK) 和药效学 (PD) 分析通常不能预测人体中的药物 PK 和 PD,并且体外 PK 和 PD 模型不提供定量 PK 参数。在这里,我们展示了人体首过药物吸收、代谢和排泄的生理 PK 模型(使用来自多个流体连接的双通道器官芯片的计算缩放数据)预测口服尼古丁的 PK 参数(使用肠道、肝脏和肾脏芯片) )和静脉注射顺铂(使用偶联的骨髓、肝脏和肾脏芯片)。这些芯片通过其内皮通道(如 Novak 等人在相关文章中报道的那样)通过常见血液替代品的顺序机器人液体传输进行连接,并共享动静脉流体混合储液器。我们还表明,顺铂 PD 的预测与之前报告的患者数据相符。通过流体耦合器官芯片定量体外到体内PK和PD参数的转换以及药物吸收、分布、代谢、排泄和毒性的预测可以改善I期临床给药方案的设计试验。











































 京公网安备 11010802027423号
京公网安备 11010802027423号