当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scientific and Regulatory Approach to Botanical Drug Development: A U.S. FDA Perspective.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.jnatprod.9b00949 Charles Wu 1 , Su-Lin Lee 2 , Cassandra Taylor 1 , Jing Li 1 , Yen-Ming Chan 1 , Rajiv Agarwal 3 , Robert Temple 4 , Douglas Throckmorton 4 , Katherine Tyner 2
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.jnatprod.9b00949 Charles Wu 1 , Su-Lin Lee 2 , Cassandra Taylor 1 , Jing Li 1 , Yen-Ming Chan 1 , Rajiv Agarwal 3 , Robert Temple 4 , Douglas Throckmorton 4 , Katherine Tyner 2
Affiliation
|
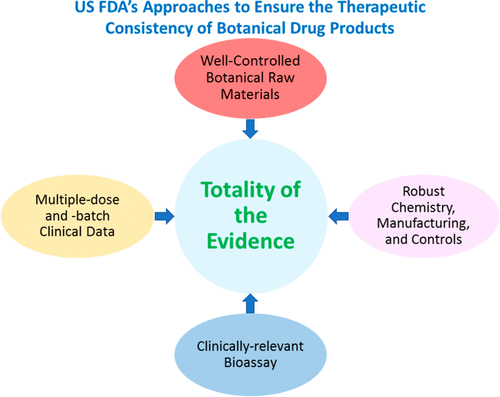
The United States FDA has received over 800 botanical investigational new drug applications (IND) and pre-IND meeting requests (PIND) in the years preceding 2018. The current data show that indications for submitted INDs cover nearly every review division of the FDA. Despite increasing global interest in the investigation of botanical mixtures as drug products, only two botanical new drug applications (NDA) have been approved in the U.S.: Veregen in 2006 and Fulyzaq (also known as Mytesi) in 2012. Given botanicals' chemical and biological complexity, efforts in characterizing their pharmacology, demonstrating therapeutic efficacy, and ensuring quality consistency remain scientific and regulatory challenges. The FDA published a revised Botanical Drug Development Guidance for Industry document in December 2016 to address developmental considerations for late-phase trials and to provide recommendations intended to facilitate botanical drug development. Herein, we present an analysis of botanical INDs showing their variety of botanical raw materials (e.g., coming from different geographic regions, single vs multiple herbs), the varied levels of previous human experience, and therapeutic areas, as well as provide an overview of experience and challenges in reviewing botanical drugs.
中文翻译:

植物药开发的科学和法规方法:美国FDA的观点。
在2018年之前的几年中,美国FDA收到了800多项植物新药申请(IND)和IND前会议要求(PIND)。当前数据显示,提交的IND适应症几乎涵盖了FDA的每个审核部门。尽管全球对研究作为药物产品的植物性混合物的兴趣日益浓厚,但美国仅批准了两种植物性新药申请(NDA):2006年的Veregen和2012年的Fulyzaq(也称为Mytesi)。考虑到植物药的化学和生物学性质复杂性,表征其药理作用,证明治疗功效以及确保质量一致性的努力仍然是科学和法规方面的挑战。FDA于2016年12月发布了修订的《工业植物药开发指南》文件,以解决后期试验的发展考量并提供旨在促进植物药开发的建议。本文中,我们对植物IND进行了分析,显示了其植物原料的种类(例如,来自不同地理区域,单一或多种草药),先前人类经历的不同水平以及治疗领域,并概述了审查植物药方面的经验和挑战。
更新日期:2020-01-26
中文翻译:

植物药开发的科学和法规方法:美国FDA的观点。
在2018年之前的几年中,美国FDA收到了800多项植物新药申请(IND)和IND前会议要求(PIND)。当前数据显示,提交的IND适应症几乎涵盖了FDA的每个审核部门。尽管全球对研究作为药物产品的植物性混合物的兴趣日益浓厚,但美国仅批准了两种植物性新药申请(NDA):2006年的Veregen和2012年的Fulyzaq(也称为Mytesi)。考虑到植物药的化学和生物学性质复杂性,表征其药理作用,证明治疗功效以及确保质量一致性的努力仍然是科学和法规方面的挑战。FDA于2016年12月发布了修订的《工业植物药开发指南》文件,以解决后期试验的发展考量并提供旨在促进植物药开发的建议。本文中,我们对植物IND进行了分析,显示了其植物原料的种类(例如,来自不同地理区域,单一或多种草药),先前人类经历的不同水平以及治疗领域,并概述了审查植物药方面的经验和挑战。











































 京公网安备 11010802027423号
京公网安备 11010802027423号