Chemosphere ( IF 8.1 ) Pub Date : 2020-01-25 , DOI: 10.1016/j.chemosphere.2020.126033 Huan Jiang 1 , Chenyuan Dang 1 , Wen Liu 2 , Ting Wang 3
|
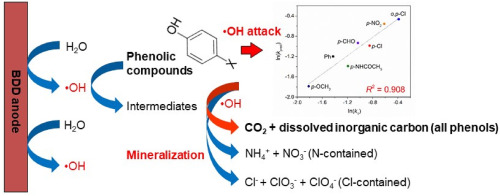
Degradation of phenols with different substituent groups (including –OCH3, –CHO, –NHCOCH3, –NO2, and −Cl) at boron-doped diamond (BDD) anodes has been studied previously based on the removal efficiency and •OH detection. Innovatively, formations of CO2 gas and various inorganic ions were examined to probe the mineralization process combined with quantitative structure-activity relationship (QSAR) analysis. As results, all phenols were efficiently degraded within 8 h with high COD removal efficiency. Three primary intermediates (hydroquinone, 1,4-benzoquinone and catechol) were identified during electrochemical oxidation and degradation pathway was proposed. More importantly, CO2 transformation efficiency ranked as: no N or Cl contained phenols (p-CHO, p-OCH3 and Ph) > N-contained phenols (p-NHCOCH3 and p-NO2) > Cl-contained phenols (p-Cl and o,p-Cl). Carbon mass balance study suggested formation of inorganic carbon (H2CO3, CO32− and HCO3−) and CO2 after organic carbon elimination. Inorganic nitrogen species (NH4+, NO3− and NO2−) and chlorine species (Cl−, ClO3− and ClO4−) were also formed after N- and Cl-contained phenols mineralization, while no volatile nitrogen species were detected. The phenols with electron-withdrawing substituents were easier to be oxidized than those with electron-donating substituents. QSAR analysis indicated that the reaction rate constant (k1) for phenols degradation was highly related to Hammett constant (∑σo,m,p) and energy gap (ELUMO - EHOMO) of the compound (R2 = 0.908), which were key parameters on evaluating the effect of structural moieties on electronic character and the chemical stability upon radical attack for a specific compound. This study presents clear evidence on mineralization mechanisms of phenols degradation at BDD anodes.
中文翻译:

硼掺杂金刚石阳极上对位取代酚的电化学氧化的自由基侵蚀和矿化机理。
基于去除效率和•OH检测,先前已研究了掺硼金刚石(BDD)阳极上具有不同取代基(包括-OCH 3,-CHO,-NHCOCH 3,-NO 2和-Cl)的苯酚的降解。。创新地检查了CO 2气体和各种无机离子的形成,以结合定量构效关系(QSAR)分析来探测矿化过程。结果,所有苯酚均在8小时内被有效降解,并具有较高的COD去除效率。提出了在电化学氧化和降解途径中鉴定出的三种主要中间体(氢醌,1,4-苯醌和邻苯二酚)。更重要的是,CO 2转化效率等级为:不包含N或Cl的酚(p -CHO,p -OCH 3和Ph)>包含N的酚(p -NHCOCH 3和p -NO 2)>包含Cl的酚(p -Cl和o ,p -Cl)。碳质量平衡研究表明形成无机碳(H 2 CO 3,CO 3 2-和HCO 3 - )和CO 2的有机碳消除之后。无机氮物质(NH 4 +,NO 3 -和NO 2- )和氯物质(CL -,CLO 3 -和C10 4 - )也之后N-和Cl-含有酚矿化而没有检测到易失性氮物种形成。具有吸电子取代基的酚比具有供电子取代基的酚更容易被氧化。QSAR分析表明反应速率常数(ķ 1)为酚降解高度相关的哈米特常数(Σ σ ø,米,p)和能隙(ë LUMO - Ë HOMO的化合物)(- [R 2 = 0.908),这是评估结构部分对电子特性的影响以及对特定化合物进行自由基攻击时化学稳定性的关键参数。这项研究提供了有关BDD阳极上苯酚降解的矿化机制的明确证据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号