当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and electrochemical investigation of lithium ions insertion processes in polyanionic compounds of lithium and transition metals
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.jelechem.2020.113894 Aleksandr V. Ivanishchev , Nelly A. Gridina , Kirill S. Rybakov , Irina A. Ivanishcheva , Ambesh Dixit
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.jelechem.2020.113894 Aleksandr V. Ivanishchev , Nelly A. Gridina , Kirill S. Rybakov , Irina A. Ivanishcheva , Ambesh Dixit
|
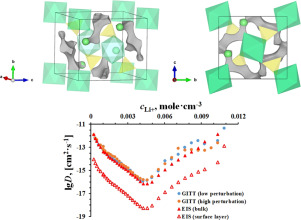
Abstract The paper considers approaches to the synthesis as well as the structural and electrochemical properties of lithium and transition metals polyanionic compounds as promising cathode materials for lithium-ion battery. The characteristics of lithium and transition metals sulfates are considered, among which iron-lithium sulfate and a composite electrode material Li2Fe(SO4)2/C based on it was selected for further detailed study. The relationships between the synthesis conditions and the structural and electrochemical characteristics of the material were revealed. Based on the structural data the computational method of the bond valence strengths (BVS) applying the bond valence energy landscape algorithm (BVEL) provided activation energy maps of lithium ion migration in the Li2Fe(SO4)2 crystal structure. The method of scanning electron microscopy (SEM) made it possible to characterize the morphological state of the material, to determine the particle size distribution, which were then used to determine the geometry of the diffusion space of particles. Applying the electrochemical kinetics investigation methods (CV, GITT, EIS) the parameters of lithium ion transport in the electrodes were determined. The quasi-equilibrium E(c) dependence of the Li2Fe(SO4)2/C electrode was obtained, this dependence was simulated using an approach based on the Frumkin intercalation isotherm and the lattice gas model, and the thermodynamic characteristics of the interparticle interaction in the material were obtained. The diffusion coefficient of lithium ions D in the material was estimated using the CV method: 1.6·10−15 cm2·s−1 in the anodic half cycle and 10−15 cm2·s−1 in the cathodic half cycle, respectively. The dependences of D on the lithium ion concentration in the intercalate and electrode potential were obtained using GITT and EIS methods, a comparison of these methods results was performed.
中文翻译:

锂和过渡金属聚阴离子化合物中锂离子嵌入过程的结构和电化学研究
摘要 本文考虑了锂和过渡金属聚阴离子化合物作为锂离子电池正极材料的合成方法以及结构和电化学性能。考虑了锂和过渡金属硫酸盐的特性,其中选择了硫酸铁锂和基于它的复合电极材料 Li2Fe(SO4)2/C 进行进一步的详细研究。揭示了合成条件与材料结构和电化学特性之间的关系。基于结构数据,键价强度 (BVS) 的计算方法应用键价能谱算法 (BVEL) 提供了 Li2Fe(SO4)2 晶体结构中锂离子迁移的活化能图。扫描电子显微镜 (SEM) 方法可以表征材料的形态状态,确定颗粒尺寸分布,然后用于确定颗粒扩散空间的几何形状。应用电化学动力学研究方法(CV、GITT、EIS)确定了电极中锂离子传输的参数。获得了 Li2Fe(SO4)2/C 电极的准平衡 E(c) 依赖性,使用基于 Frumkin 插层等温线和晶格气体模型的方法模拟了这种依赖性,以及粒子间相互作用的热力学特性获得了材料。锂离子在材料中的扩散系数D采用CV法估算:1。阳极半周期为 6·10-15 cm2·s-1,阴极半周期为 10-15 cm2·s-1。使用 GITT 和 EIS 方法获得了 D 对插层中锂离子浓度和电极电位的依赖性,并对这些方法的结果进行了比较。
更新日期:2020-03-01
中文翻译:

锂和过渡金属聚阴离子化合物中锂离子嵌入过程的结构和电化学研究
摘要 本文考虑了锂和过渡金属聚阴离子化合物作为锂离子电池正极材料的合成方法以及结构和电化学性能。考虑了锂和过渡金属硫酸盐的特性,其中选择了硫酸铁锂和基于它的复合电极材料 Li2Fe(SO4)2/C 进行进一步的详细研究。揭示了合成条件与材料结构和电化学特性之间的关系。基于结构数据,键价强度 (BVS) 的计算方法应用键价能谱算法 (BVEL) 提供了 Li2Fe(SO4)2 晶体结构中锂离子迁移的活化能图。扫描电子显微镜 (SEM) 方法可以表征材料的形态状态,确定颗粒尺寸分布,然后用于确定颗粒扩散空间的几何形状。应用电化学动力学研究方法(CV、GITT、EIS)确定了电极中锂离子传输的参数。获得了 Li2Fe(SO4)2/C 电极的准平衡 E(c) 依赖性,使用基于 Frumkin 插层等温线和晶格气体模型的方法模拟了这种依赖性,以及粒子间相互作用的热力学特性获得了材料。锂离子在材料中的扩散系数D采用CV法估算:1。阳极半周期为 6·10-15 cm2·s-1,阴极半周期为 10-15 cm2·s-1。使用 GITT 和 EIS 方法获得了 D 对插层中锂离子浓度和电极电位的依赖性,并对这些方法的结果进行了比较。









































 京公网安备 11010802027423号
京公网安备 11010802027423号