当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interactions between Viperin, Vesicle-Associated Membrane Protein A, and Hepatitis C Virus Protein NS5A Modulate Viperin Activity and NS5A Degradation.
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.biochem.9b01090 Soumi Ghosh , Ayesha M. Patel , Timothy J. Grunkemeyer , Arti B. Dumbrepatil , Kelcie Zegalia , Robert T. Kennedy , E. Neil G. Marsh
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.biochem.9b01090 Soumi Ghosh , Ayesha M. Patel , Timothy J. Grunkemeyer , Arti B. Dumbrepatil , Kelcie Zegalia , Robert T. Kennedy , E. Neil G. Marsh
|
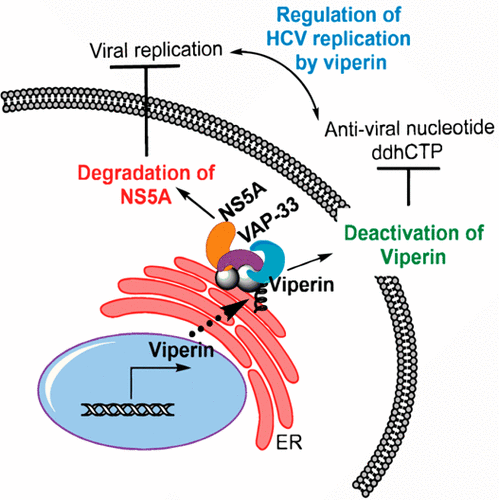
The radical SAM enzyme, viperin, exerts a wide range of antiviral effects through both the synthesis of the antiviral nucleotide 3'-deoxy-3',4'-didehydro-CTP (ddhCTP) and through its interactions with various cellular and viral proteins. Here we investigate the interaction of viperin with hepatitis C virus nonstructural protein 5A (NS5A) and the host sterol regulatory protein, vesicle-associated membrane protein A (VAP-33). NS5A and VAP-33 form part of the viral replication complex that is essential for replicating the RNA genome of the hepatitis C virus. Using transfected enzymes in HEK293T cells, we show that viperin binds independently to both NS5A and the C-terminal domain of VAP-33 (VAP-33C) and that this interaction is dependent on the proteins being colocalized to the ER membrane. Coexpression of VAP-33C and NS5A resulted in changes to the catalytic activity of viperin that depended upon viperin being colocalized to the ER membrane. The viperin-NS5A-VAP-33C complex exhibited the lowest specific activity, indicating that NS5A may inhibit viperin's ability to synthesize ddhCTP. Coexpression of viperin with NS5A was also found to significantly reduce cellular NS5A levels, most likely by increasing the rate of proteasomal degradation. An inactive mutant of viperin, unable to bind the iron-sulfur cluster, was similarly effective at reducing cellular NS5A levels.
中文翻译:

Viperin、囊泡相关膜蛋白 A 和丙型肝炎病毒蛋白 NS5A 之间的相互作用调节 Viperin 活性和 NS5A 降解。
自由基 SAM 酶 viperin 通过合成抗病毒核苷酸 3'-deoxy-3',4'-didehydro-CTP (ddhCTP) 及其与各种细胞和病毒蛋白的相互作用发挥广泛的抗病毒作用。在这里,我们研究 viperin 与丙型肝炎病毒非结构蛋白 5A (NS5A) 和宿主甾醇调节蛋白、囊泡相关膜蛋白 A (VAP-33) 的相互作用。NS5A 和 VAP-33 构成病毒复制复合物的一部分,该复合物对于复制丙型肝炎病毒的 RNA 基因组至关重要。使用 HEK293T 细胞中的转染酶,我们显示毒蛇素独立地结合 NS5A 和 VAP-33 (VAP-33C) 的 C 端结构域,并且这种相互作用取决于共定位到 ER 膜的蛋白质。VAP-33C 和 NS5A 的共表达导致 viperin 的催化活性发生变化,这取决于 viperin 共定位到 ER 膜上。viperin-NS5A-VAP-33C 复合物表现出最低的比活性,表明 NS5A 可能抑制 viperin 合成 ddhCTP 的能力。还发现毒蛇素与 NS5A 的共表达显着降低了细胞 NS5A 水平,很可能是通过增加蛋白酶体降解的速率。viperin 的无活性突变体无法结合铁硫簇,在降低细胞 NS5A 水平方面同样有效。还发现毒蛇素与 NS5A 的共表达显着降低了细胞 NS5A 水平,很可能是通过增加蛋白酶体降解的速率。viperin 的无活性突变体无法结合铁硫簇,在降低细胞 NS5A 水平方面同样有效。还发现毒蛇素与 NS5A 的共表达显着降低了细胞 NS5A 水平,很可能是通过增加蛋白酶体降解的速率。viperin 的无活性突变体无法结合铁硫簇,在降低细胞 NS5A 水平方面同样有效。
更新日期:2020-01-31
中文翻译:

Viperin、囊泡相关膜蛋白 A 和丙型肝炎病毒蛋白 NS5A 之间的相互作用调节 Viperin 活性和 NS5A 降解。
自由基 SAM 酶 viperin 通过合成抗病毒核苷酸 3'-deoxy-3',4'-didehydro-CTP (ddhCTP) 及其与各种细胞和病毒蛋白的相互作用发挥广泛的抗病毒作用。在这里,我们研究 viperin 与丙型肝炎病毒非结构蛋白 5A (NS5A) 和宿主甾醇调节蛋白、囊泡相关膜蛋白 A (VAP-33) 的相互作用。NS5A 和 VAP-33 构成病毒复制复合物的一部分,该复合物对于复制丙型肝炎病毒的 RNA 基因组至关重要。使用 HEK293T 细胞中的转染酶,我们显示毒蛇素独立地结合 NS5A 和 VAP-33 (VAP-33C) 的 C 端结构域,并且这种相互作用取决于共定位到 ER 膜的蛋白质。VAP-33C 和 NS5A 的共表达导致 viperin 的催化活性发生变化,这取决于 viperin 共定位到 ER 膜上。viperin-NS5A-VAP-33C 复合物表现出最低的比活性,表明 NS5A 可能抑制 viperin 合成 ddhCTP 的能力。还发现毒蛇素与 NS5A 的共表达显着降低了细胞 NS5A 水平,很可能是通过增加蛋白酶体降解的速率。viperin 的无活性突变体无法结合铁硫簇,在降低细胞 NS5A 水平方面同样有效。还发现毒蛇素与 NS5A 的共表达显着降低了细胞 NS5A 水平,很可能是通过增加蛋白酶体降解的速率。viperin 的无活性突变体无法结合铁硫簇,在降低细胞 NS5A 水平方面同样有效。还发现毒蛇素与 NS5A 的共表达显着降低了细胞 NS5A 水平,很可能是通过增加蛋白酶体降解的速率。viperin 的无活性突变体无法结合铁硫簇,在降低细胞 NS5A 水平方面同样有效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号