当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen Evolution Activity on NiOOH Catalysts: Four-Coordinated Ni Cation as the Active Site and the Hydroperoxide Mechanism
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-02-06 , DOI: 10.1021/acscatal.9b04975 Li-Fen Li 1 , Ye-Fei Li 1 , Zhi-Pan Liu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-02-06 , DOI: 10.1021/acscatal.9b04975 Li-Fen Li 1 , Ye-Fei Li 1 , Zhi-Pan Liu 1
Affiliation
|
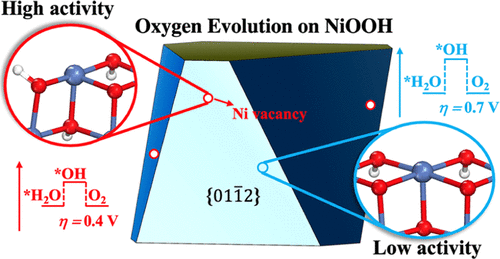
The NiOOH catalyst as obtained dynamically from electrodeposition of Ni2+(aq) in the borate-containing electrolyte was observed to exhibit much higher oxygen evolution activity at a near-neutral pH range (7–9) compared to other NiOx-based materials. Here, we demonstrate that this intriguing high activity is owing to the high concentration of Ni cationic vacancy on the nascent ultra-small NiOOH particles (<3 nm). By using first-principles calculations, we compute the thermodynamics of Ni dissolution and clarify the mechanism of oxygen evolution reaction (OER) on the γ-NiOOH surface. We show that (i) ∼4% Ni cations on the surface of γ-NiOOH dissolve at pH = 7 and 1.73 V versus reversible hydrogen electrode; (ii) on the pristine γ-NiOOH surface, OER proceeds via the “lattice peroxide” mechanism (*H2O → *OH → *O–OlattH* → O–Olatt → O2) with an overpotential of 0.70 V; (iii) in the presence of Ni cationic vacancies, OER proceeds via the “hydroperoxide” mechanism (*OH + *H2O → *2OH → *OOH → O2) with an overpotential of 0.40 V. Our electronic structure and geometrical structure analyses demonstrate that the structural flexibility at the four-coordinated Ni site nearby Ni vacancy, featuring the ability to bind two terminal oxo species, is key to boost the activity. Considering the presence of the active OOH intermediate, our theory thus implies that the ultra-small oxide nanoclusters with ample cation vacancies could be a paradigm in catalyst design for oxidation reactions.
中文翻译:

NiOOH催化剂上的析氧活性:四配位Ni阳离子为活性位点和氢过氧化物机理
与其他NiO x相比,通过在含硼酸盐的电解质中电沉积Ni 2+(水溶液)动态获得的NiOOH催化剂在近中性pH范围(7–9)表现出更高的氧释放活性。基材料。在这里,我们证明了这种引人入胜的高活性是由于新生的超小NiOOH颗粒(<3 nm)上的Ni阳离子空位浓度很高。通过第一性原理计算,我们计算了镍溶解的热力学,并阐明了γ-NiOOH表面上的析氧反应(OER)的机理。我们发现(i)相对于可逆的氢电极,在pH = 7和1.73 V时,γ-NiOOH表面有〜4%的Ni阳离子溶解;(ii)在原始的γ-NiOOH表面上,OER通过“过氧化晶格”机理(* H 2 O→* OH→* O–O latt H *→O–O latt →O 2进行))的过电位为0.70 V; (iii)在存在镍阳离子空位的情况下,OER通过“氢过氧化物”机理(* OH + * H 2 O→* 2OH→* OOH→O 2)进行处理,超电势为0.40V。我们的电子结构和几何结构分析表明,镍空位附近四配位镍位点的结构柔性具有结合两个末端氧代物种的能力,是提高活性的关键。考虑到活性OOH中间体的存在,我们的理论因此暗示具有充足阳离子空位的超小氧化物纳米簇可能是氧化反应催化剂设计的范例。
更新日期:2020-02-06
中文翻译:

NiOOH催化剂上的析氧活性:四配位Ni阳离子为活性位点和氢过氧化物机理
与其他NiO x相比,通过在含硼酸盐的电解质中电沉积Ni 2+(水溶液)动态获得的NiOOH催化剂在近中性pH范围(7–9)表现出更高的氧释放活性。基材料。在这里,我们证明了这种引人入胜的高活性是由于新生的超小NiOOH颗粒(<3 nm)上的Ni阳离子空位浓度很高。通过第一性原理计算,我们计算了镍溶解的热力学,并阐明了γ-NiOOH表面上的析氧反应(OER)的机理。我们发现(i)相对于可逆的氢电极,在pH = 7和1.73 V时,γ-NiOOH表面有〜4%的Ni阳离子溶解;(ii)在原始的γ-NiOOH表面上,OER通过“过氧化晶格”机理(* H 2 O→* OH→* O–O latt H *→O–O latt →O 2进行))的过电位为0.70 V; (iii)在存在镍阳离子空位的情况下,OER通过“氢过氧化物”机理(* OH + * H 2 O→* 2OH→* OOH→O 2)进行处理,超电势为0.40V。我们的电子结构和几何结构分析表明,镍空位附近四配位镍位点的结构柔性具有结合两个末端氧代物种的能力,是提高活性的关键。考虑到活性OOH中间体的存在,我们的理论因此暗示具有充足阳离子空位的超小氧化物纳米簇可能是氧化反应催化剂设计的范例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号