当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst Design for Alkene Epoxidation by Molecular Analogues of Heterogeneous Titanium-Silicalite Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-24 , DOI: 10.1021/acscatal.9b05147 Albert Solé-Daura 1 , Teng Zhang 2 , Hugo Fouilloux 2, 3 , Carine Robert 3 , Christophe M. Thomas 3 , Lise-Marie Chamoreau 2 , Jorge J. Carbó 1 , Anna Proust 2 , Geoffroy Guillemot 2 , Josep M. Poblet 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-24 , DOI: 10.1021/acscatal.9b05147 Albert Solé-Daura 1 , Teng Zhang 2 , Hugo Fouilloux 2, 3 , Carine Robert 3 , Christophe M. Thomas 3 , Lise-Marie Chamoreau 2 , Jorge J. Carbó 1 , Anna Proust 2 , Geoffroy Guillemot 2 , Josep M. Poblet 1
Affiliation
|
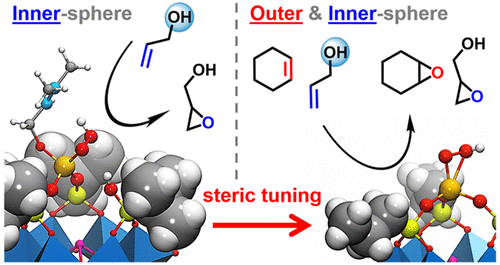
The epoxidation of allylic alcohols with H2O2 catalyzed by the hybrid [α-B-SbW9O33(tBuSiO)3Ti(OiPr)]3– (1) anion as a molecular model of heterogeneous Ti-silicalite TS-1 catalyst was analyzed by means of DFT to determine the main factors that control the catalytic process and, finally, to improve the value of the available catalysts. Our calculations revealed that unlike other alkenes, allylic alcohols can bind the Ti center after activation of the precatalyst via hydrolysis to give the corresponding Ti-alcoholate, which is the catalyst resting state. Next, the dissociative addition of hydrogen peroxide to Ti causes the cleavage of a Ti–OSi junction to form a Ti(η2-OOH) moiety. The partial detachment of the Ti from the catalyst structure yields an intermediate with a flexible Ti center from which the Ti-OOH group can transfer an electrophilic oxygen to the alkene substrate in an inner-sphere fashion. The rate-determining process, which involves the heterolytic activation of H2O2 over the Ti(IV) and the electrophilic O-transfer, accounts for an overall free-energy barrier of 23.0 kcal mol–1 for 2-methyl-2-buten-1-ol, in line with the experimental value of 22.3. Conversely, the outer-sphere O-transfer—also accessible to nonfunctionalized alkenes—occurs through a more strained transition state that lays above in energy (by ∼4 kcal mol–1), giving a clue to explain the low yields reported experimentally for nonfunctionalized olefins. We also found that reducing the bulkiness of the substituents in the silanol functions of the catalyst has a positive influence on the catalytic activity, decreasing the overall free-energy barriers for the outer-sphere path. With this knowledge, we developed other catalytic species with tailored steric properties based on [SbW9O33(RSiOH)3]3– structure (R=iPr and nPr), which were synthesized, characterized, and successfully applied to the catalytic epoxidation of unfunctionalized alkenes. Present results clearly show that the detailed knowledge of the reaction mechanisms, even for complex processes, is possible nowadays and that the acquired information allows designing catalysts with desired activities.
中文翻译:

非均相钛硅沸石催化剂分子模拟研究烯烃环氧化催化剂的设计
烯丙基醇的用H环氧化2 Ó 2由混合催化[α-B-线控9 Ö 33(吨BuSiO)3的Ti(O我PR)] 3-(1通过DFT分析阴离子作为多相Ti-silicalite TS-1催化剂的分子模型,以确定控制催化过程的主要因素,并最终提高可用催化剂的价值。我们的计算表明,与其他烯烃不同,烯丙醇可以通过水解将预催化剂活化后生成相应的钛醇盐,该钛中心是催化剂的静止状态。接着,游离的加入过氧化氢与Ti引起的Ti-OSI结,以形成由Ti(η的裂解2 -OOH)部分。Ti从催化剂结构中部分脱离,产生了具有柔性Ti中心的中间体,Ti-OOH基团可以从该中间体将内含氧转移亲电子到烯烃基体中时尚。速率决定过程,它涉及H的异裂活化2 ö 2在该Ti(IV)和亲电O型转移,占23.0千卡mol的总自由能屏障-1 2-甲基-2- buten-1-ol,符合实验值22.3。相反,外层O转移(也可通过非官能化烯烃获得)发生在能量之上(通过〜4 kcal mol –1),处于更高的应变状态,从而提供了线索来解释实验报道的低官能度低收率。烯烃。我们还发现减少体积催化剂的硅烷醇官能团中的取代基的一部分对催化活性具有积极影响,从而降低了外球径的总自由能垒。借助这些知识,我们基于[SbW 9 O 33(R SiOH)3 ] 3–结构(R = i Pr和nPr),合成,表征并成功地用于未官能化烯烃的催化环氧化。目前的结果清楚地表明,如今,即使对于复杂的方法,也可以详细了解反应机理,并且所获得的信息可以设计出具有所需活性的催化剂。
更新日期:2020-01-24
中文翻译:

非均相钛硅沸石催化剂分子模拟研究烯烃环氧化催化剂的设计
烯丙基醇的用H环氧化2 Ó 2由混合催化[α-B-线控9 Ö 33(吨BuSiO)3的Ti(O我PR)] 3-(1通过DFT分析阴离子作为多相Ti-silicalite TS-1催化剂的分子模型,以确定控制催化过程的主要因素,并最终提高可用催化剂的价值。我们的计算表明,与其他烯烃不同,烯丙醇可以通过水解将预催化剂活化后生成相应的钛醇盐,该钛中心是催化剂的静止状态。接着,游离的加入过氧化氢与Ti引起的Ti-OSI结,以形成由Ti(η的裂解2 -OOH)部分。Ti从催化剂结构中部分脱离,产生了具有柔性Ti中心的中间体,Ti-OOH基团可以从该中间体将内含氧转移亲电子到烯烃基体中时尚。速率决定过程,它涉及H的异裂活化2 ö 2在该Ti(IV)和亲电O型转移,占23.0千卡mol的总自由能屏障-1 2-甲基-2- buten-1-ol,符合实验值22.3。相反,外层O转移(也可通过非官能化烯烃获得)发生在能量之上(通过〜4 kcal mol –1),处于更高的应变状态,从而提供了线索来解释实验报道的低官能度低收率。烯烃。我们还发现减少体积催化剂的硅烷醇官能团中的取代基的一部分对催化活性具有积极影响,从而降低了外球径的总自由能垒。借助这些知识,我们基于[SbW 9 O 33(R SiOH)3 ] 3–结构(R = i Pr和nPr),合成,表征并成功地用于未官能化烯烃的催化环氧化。目前的结果清楚地表明,如今,即使对于复杂的方法,也可以详细了解反应机理,并且所获得的信息可以设计出具有所需活性的催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号