Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2020-01-25 , DOI: 10.1016/j.jfluchem.2020.109468 Seiji Tsuzuki , Tadafumi Uchimaru
|
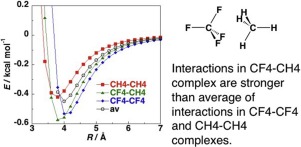
The CCSD(T) level intermolecular interaction energies calculated for the CF4-CH4 complex are compared with those for the CF4-CF4 and CH4-CH4 complexes. The interactions in the CF4-CH4 complex is stronger than the average of the interactions in the CF4-CF4 and CH4-CH4 complexes. DFT-SAPT calculations show that the dispersion interactions in the CF4-CH4 complex, which are the primary source of the attraction, are nearly identical to the average of those in the CF4-CF4 and CH4-CH4 complexes, while the attractive electrostatic interactions in the CF4-CH4 complex is stronger than the average of those in the CF4-CF4 and CH4-CH4 complexes.
中文翻译:

在CF的吸引力大小4个-CH 4相互作用:是CF 4个-CH 4相互作用比平均CF弱4 -CF 4和CH 4 -CH 4个相互作用?
将针对CF 4 -CH 4络合物计算出的CCSD(T)级分子间相互作用能与针对CF 4 -CF 4和CH 4 -CH 4络合物计算得出的值进行比较。CF 4 -CH 4络合物中的相互作用强于CF 4 -CF 4和CH 4 -CH 4络合物中的相互作用的平均值。DFT-SAPT计算表明,在CF的分散的相互作用4 -CH 4复合物,其是所述游览项目的主要来源,是几乎相同的平均那些在CF的4-CF 4和CH 4种-CH 4复合物,而在CF的有吸引力的静电相互作用4 -CH 4配合物是在CF比一般的那些强4 -CF 4和CH 4 -CH 4个络合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号