Molecular Catalysis ( IF 3.9 ) Pub Date : 2020-01-25 , DOI: 10.1016/j.mcat.2020.110774 Hanaa Mansour , Morad M. El-Hendawy
|
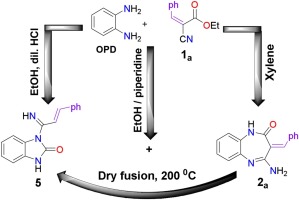
This work introduced new members to the pharmaceutical family of 1,5-benzodiazepin-2-ones, 2a–e. These compounds were synthesized in moderate yields via the reaction of 1,2-bifunctional substrates (α-cyanocinnamates) with o-phenylenediamine in xylene. The conduction of the above reaction in the presence of piperidine has produced amazing product, N-alkenylimidazolone derivative (5), in addition to the traditional one, 1,5-benzodiazepin-2-one (2a) in 3:1 ratio. The density functional theory (DFT) could successfully explain the role of piperidine as an organic catalyst to produce both products in this ratio through ethanol-assisted mechanisms. It is also amazing that we could obtain 5 solely by the dry fusion of 2a through the thermal contraction of diazepinone ring into imidazolone one. The mechanism of diazepinone-imidazolone transformation was proposed and validated by the DFT calculations. The findings showed that the precise proton transfer of primary amino hydrogen of 2a is the play-maker in the reaction game. The proposed mechanisms of the three transformations can be useful for investigation of the formation and deformation of other 1,5-diazepine systems.
中文翻译:

哌啶催化合成1,5-苯并二氮杂-2-酮的机理研究
这项工作为1,5-苯并二氮杂-2-酮2 a-e的药物家族引入了新成员。这些化合物是通过1,2-双官能底物(α-氰基肉桂酸酯)与邻苯二胺在二甲苯中的反应以中等收率合成的。在哌啶存在下进行上述反应,除产生传统的1,5-苯并二氮杂-2--2-酮(2 a)外,还产生了惊人的产物N-烯基咪唑酮衍生物(5 )以3:1的比例。密度泛函理论(DFT)可以成功地解释哌啶作为有机催化剂通过乙醇辅助机理以该比例产生两种产物的作用。同样令人惊奇的是,我们可以通过将二氮杂pin酮环热收缩成咪唑酮一,仅通过2 a的干融合来获得5。提出了二氮杂pin酮-咪唑啉酮转化的机理,并通过DFT计算对其进行了验证。研究结果表明,2 a伯氨基氢的精确质子转移是反应博弈的推动者。提出的三种转化机制可用于研究其他1,5-二氮杂systems系统的形成和变形。











































 京公网安备 11010802027423号
京公网安备 11010802027423号