Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-01-24 , DOI: 10.1016/j.jcat.2020.01.004 Christoph Göbel , Stefan Schmidt , Christian Froese , Qi Fu , Yen-Ting Chen , Qiushi Pan , Martin Muhler
|
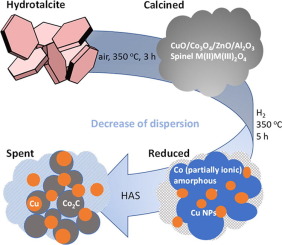
Bimetallic Co-Cu catalysts are widely applied in higher alcohol synthesis (HAS), but the formation of the final active structure has not yet been fully clarified, especially for Co-rich catalysts. We investigated the structural evolution of a Co-Cu catalyst (Co:Cu = 2) from the hydrotalcite precursor containing additional Al3+ and Zn2+ to the final active state after 80 h under reaction conditions at 280 °C and 60 bar. The reconstruction of the bimetallic Co-Cu nanoparticles obtained by H2 reduction was induced by the feed gas consisting of an equimolar H2 and CO syngas mixture resulting in fast phase separation and sintering of metallic Cu0 and Co0 in the first 2 h time on stream (TOS) and a continuous carbidization of Co0 forming Co2C and its sintering until steady state was reached after 40 h TOS. An intergrowth of metallic Cu0 nanoparticles with Co2C nanoparticles was observed to occur under reaction conditions. The high selectivity to oxygenates amounting to 41% compared with 29% to hydrocarbons is ascribed to the multi-functional Co2C/Cu0 interface enabling dissociative CO adsorption, hydrogenation and CO insertion. The formation of hydrogenated carbon species (CxHy) originating from dissociative CO chemisorption is assumed to be favored by hydrogen spillover from Cu0 to Co2C. The adsorption sites for molecular CO provided by both Cu0 and Co2C facilitate its insertion into the CxHy intermediates thus leading to a higher selectivity to alcohols following the Anderson-Schulz-Flory distribution.
中文翻译:

在高压下CO加氢成高级醇的双金属Co-Cu催化剂的结构演变
双金属Co-Cu催化剂广泛用于高级醇合成(HAS),但最终活性结构的形成尚未完全阐明,特别是对于富Co催化剂。我们研究了在280°C和60 bar的反应条件下80 h后,Co-Cu催化剂(Co:Cu = 2)从含有额外Al 3+和Zn 2+的水滑石前体的结构演变为最终活性态的过程。由H 2还原获得的双金属Co-Cu纳米颗粒的重构是由由等摩尔H 2和CO合成气混合物组成的进料气引起的,从而实现了金属Cu 0和Co 0的快速相分离和烧结。在最初的2小时运行时间(TOS)中,Co 0连续碳化形成Co 2 C并烧结,直到40 h TOS之后达到稳定状态。在反应条件下观察到金属Cu 0纳米颗粒与Co 2 C纳米颗粒的共生。多官能团的Co 2 C / Cu 0界面使含氧化合物的选择性高达41%,而对烃类的选择性为29%,可实现离解性CO吸附,氢化和CO的插入。假定由于铜的氢溢出,有利于源自解离性CO化学吸附的氢化碳物质(C x H y)的形成。0对Co 2由铜既提供C的吸附位点的分子CO 0和Co 2 ç便于其插入到C X ħ ý中间体从而导致更高的选择性为醇继安德森-舒尔茨-弗洛里分布。











































 京公网安备 11010802027423号
京公网安备 11010802027423号