Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-01-25 , DOI: 10.1016/j.jcat.2019.12.043 Stephanie Kwon , Ting Chun Lin , Enrique Iglesia
|
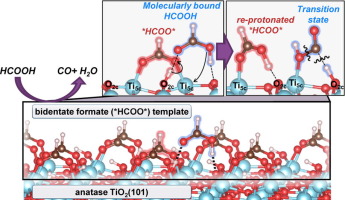
Mechanistic details of HCOOH decomposition routes provide valuable insights into reactions involving bound formates as intermediates or spectators; these routes are also widely used as a probe of the acid-base properties of oxide surfaces. The identity and kinetic relevance of bound intermediates, transition states, and elementary steps are reported here for HCOOH dehydration on anatase and rutile TiO2 surfaces through complementary kinetic, isotopic, spectroscopic and theoretical assessments. Five-coordinate exposed Ti5c centers are saturated with bidentate formates (*HCOO*) at catalytic conditions (423–463 K; 0.1–3 kPa HCOOH), as evident from infrared spectra collected during catalysis and the amounts of HCOOH and CO evolved upon heating the TiO2 samples containing pre-adsorbed HCOOH-derived species. These *HCOO* species are inactive but form a stable “surface template” that contains stochiometric protons onto which HCOOH binds molecularly (HCOOH-H*) to form a coexisting adlayer. H2O elimination from HCOOH-H* is the sole kinetically-relevant step. DFT-derived barriers show that this step involves its reaction with Ti5c-O2c that acts as a Lewis acid-base pair. Such route, in turn, requires the access of HCOOH-H* to a Ti5c center, which is made available through a momentary reprotonation of a *HCOO*. This step is much less facile on rutile than on anatase due to stronger acid strength of its Ti5c centers that binds *HCOO* species more strongly and its shorter Ti5c-Ti5c distances that induce greater repulsions between co-adsorbed HCOOH* formed upon reprotonation step. These differences account for low dehydration reactivity of rutile at these temperatures. This mechanistic interpretation is in full accord with DFT-derived barriers, binding energies, and kinetic isotope effects that quantitatively agree with the values from regressed kinetic and thermodynamic parameters, with in-situ infrared spectra that identify HCOOH-H* species as the sole reactive intermediates, and with the differences in turnover rates between anatase and rutile catalysts. These dehydration routes are also consistent with the surface chemistry expected for Lewis acid-base pairs on stoichiometry TiO2 surfaces without requiring the presence or involvement of reduced centers or titanols in the catalytic cycle. The reaction routes described in this work show how strongly-bound species, evident in presence and unreactive nature from in-situ infrared spectra, provide an organic “permanent” template for reactions of weakly-bound species that are often invisible in spectroscopy.
中文翻译:

锐钛矿和金红石TiO 2表面上甲酸脱水反应的基本步骤和位置要求
HCOOH分解路线的机理细节为涉及作为中间体或旁观者的结合甲酸酯的反应提供了有价值的见解;这些途径也被广泛用作氧化物表面酸碱性质的探针。通过互补的动力学,同位素,光谱和理论评估,此处报道了锐钛矿和金红石TiO 2表面上HCOOH脱水的键合中间体,过渡态和基本步骤的同一性和动力学相关性。五坐标暴露的Ti 5c中心在催化条件(423–463 K; 0.1–3 kPa HCOOH)下被二齿甲酸盐(* HCOO *)饱和,这从催化过程中收集到的红外光谱以及随着加热TiO 2包含预先吸附的HCOOH衍生物种的样品。这些* HCOO *物种没有活性,但形成了稳定的“表面模板”,其中包含化学计量的质子,HCOOH在分子上结合(HCOOH-H *)以形成共存的夹层。从HCOOH-H *中消除H 2 O是唯一与动力学相关的步骤。DFT衍生的势垒表明该步骤涉及其与充当路易斯酸碱对的Ti 5c -O 2c的反应。这样的路线又需要HCOOH-H *进入Ti 5c中心,该中心可通过* HCOO *的瞬时质子化获得。由于其Ti 5c具有更强的酸强度,因此该步骤对金红石的反应比对锐钛矿的反应少得多。中心更牢固地结合* HCOO *物种,其较短的Ti 5c -Ti 5c距离在质子化步骤形成的共吸附HCOOH *之间引起更大的排斥力。这些差异解释了在这些温度下金红石的低脱水反应性。这种机理上的解释完全符合DFT衍生的势垒,结合能和动力学同位素效应,这些效应与原位回归的动力学和热力学参数的值定量地一致红外光谱确定HCOOH-H *是唯一的反应性中间体,并且锐钛矿型和金红石型催化剂的周转率不同。这些脱水途径也与化学计量的TiO 2表面上的路易斯酸-碱对所期望的表面化学相一致,而无需在催化循环中存在或不涉及还原的中心或二烷醇。这项工作中描述的反应路线表明,存在于现场且从原位红外光谱中发现无反应性的强结合物种如何为弱结合物种的反应提供有机“永久”模板,而弱结合物种在光谱学中通常是不可见的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号