当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Equilibrium data and thermodynamic studies of L-tryptophan partition in alcohol / phosphate potassium salt-based aqueous two phase systems
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jct.2020.106048 Seyyed Mohammad Arzideh , Kamyar Movagharnejad , Mohsen Pirdashti
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jct.2020.106048 Seyyed Mohammad Arzideh , Kamyar Movagharnejad , Mohsen Pirdashti
|
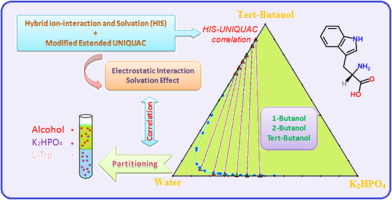
Abstract Experimental phase diagrams for {1-butanol/2-butanol/t-butyl alcohol + potassium hydrogen phosphate (K2HPO4) + water} at 298.15 K have been determined, and their ability to separate an amino acid, L-Tryptophan, were measured. An excess Gibbs energy model has been proposed for modelling of aqueous two-phase systems (ATPS) containing light straight/branched alcohol, K2HPO4, and water. The hybrid ion-interaction and solvation model (HIS) was implemented to calculate the long-range ion-ion and middle-range ion-solvent interactions, and the UNIQUAC model was used for the short-range solvent-solvent interactions. The continuum characteristics such as density, dielectric constant, and solvation parameters were considered as mixed-solvent property dependent correlations. The proposed excess Gibbs energy model, HIS-UNIQUAC, has been found to describe the LLE data in a satisfactory precision of less than 0.331% for mass percent of experimental data. Moreover, the partitioning coefficient shows a perfect correlation (R2 > 0.982) with the long and middle range term of excess Gibbs energy model.
中文翻译:

L-色氨酸在醇/磷酸钾盐基水相两相系统中分配的平衡数据和热力学研究
摘要 已确定 {1-丁醇/2-丁醇/叔丁醇 + 磷酸氢钾 (K2HPO4) + 水} 在 298.15 K 的实验相图,并测量了它们分离氨基酸 L-色氨酸的能力. 已经提出了一种过量 Gibbs 能量模型,用于对含有轻质直链/支链醇、K2HPO4 和水的含水两相系统 (ATPS) 进行建模。实施混合离子相互作用和溶剂化模型 (HIS) 来计算长程离子-离子和中程离子-溶剂相互作用,并使用 UNIQUAC 模型计算短程溶剂-溶剂相互作用。连续介质特性如密度、介电常数和溶剂化参数被认为是混合溶剂特性相关性。提议的过剩吉布斯能量模型,HIS-UNIQUAC,已经发现以小于 0.331% 的实验数据质量百分比的令人满意的精度描述 LLE 数据。此外,分配系数与超量吉布斯能量模型的中长期项显示出完美的相关性(R2 > 0.982)。
更新日期:2020-05-01
中文翻译:

L-色氨酸在醇/磷酸钾盐基水相两相系统中分配的平衡数据和热力学研究
摘要 已确定 {1-丁醇/2-丁醇/叔丁醇 + 磷酸氢钾 (K2HPO4) + 水} 在 298.15 K 的实验相图,并测量了它们分离氨基酸 L-色氨酸的能力. 已经提出了一种过量 Gibbs 能量模型,用于对含有轻质直链/支链醇、K2HPO4 和水的含水两相系统 (ATPS) 进行建模。实施混合离子相互作用和溶剂化模型 (HIS) 来计算长程离子-离子和中程离子-溶剂相互作用,并使用 UNIQUAC 模型计算短程溶剂-溶剂相互作用。连续介质特性如密度、介电常数和溶剂化参数被认为是混合溶剂特性相关性。提议的过剩吉布斯能量模型,HIS-UNIQUAC,已经发现以小于 0.331% 的实验数据质量百分比的令人满意的精度描述 LLE 数据。此外,分配系数与超量吉布斯能量模型的中长期项显示出完美的相关性(R2 > 0.982)。









































 京公网安备 11010802027423号
京公网安备 11010802027423号