当前位置:
X-MOL 学术
›
Chem. Geol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroperoxo [Fe(III)-OOH] and ferryl-oxo [Fe(IV)=O] oxidative species involved in As(III) oxidation catalyzed by pyrite under alkaline conditions
Chemical Geology ( IF 3.6 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.chemgeo.2020.119480 Shi-Wei Sun , Song-Hai Wu , Zi-He Meng , Xiao-Cong Zhang , Shi-Jie Wang , Xiang-Ming Wang , Yong Liu , Hai-Tao Ren , Shao-Yi Jia , Ke-Xin Yao , He Bai , Xu Han
Chemical Geology ( IF 3.6 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.chemgeo.2020.119480 Shi-Wei Sun , Song-Hai Wu , Zi-He Meng , Xiao-Cong Zhang , Shi-Jie Wang , Xiang-Ming Wang , Yong Liu , Hai-Tao Ren , Shao-Yi Jia , Ke-Xin Yao , He Bai , Xu Han
|
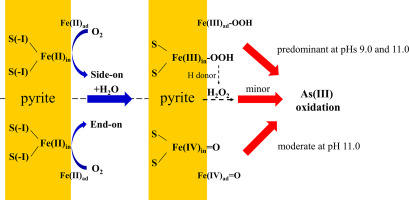
Abstract Pyrite is an extensively-distributed mineral that catalyzes oxidation of As(III) to As(V) by dissolved oxygen (DO) in natural environments; however, the catalytic mechanism of pyrite under alkaline conditions is still not clear. In this study, we observed increased oxidation rates of soluble As(III) with increasing pHs under alkaline conditions (7.5-11.0). ESR analysis and quenching experiments rule out contributions of •OH, O2•- and 1O2 to As(III) oxidation, and indicate that Fe(III)-OOH and/or H2O2 are indeed the primary oxidative species at pHs 9.0 and 11.0, and Fe(IV)=O also contributes at pH 11.0. Selective oxidation of a series of organic substrates to the corresponding oxidation products confirms the presence of Fe(III)-OOH and/or Fe(IV)=O in the pyrite system in alkaline solutions or organic solvents. Although the addition of glucose apparently increases the production of H2O2 at pHs 9.0 and 11.0, such an increase does not apparently accelerate the oxidation rate of As(III), implying that H2O2 is not the predominant species in the oxidation of As(III). This study provides new insight into the oxidative species in the pyrite-DO system under alkaline conditions.
中文翻译:

Hydroperoxo [Fe(III)-OOH] 和ferryl-oxo [Fe(IV)=O] 氧化物质参与黄铁矿在碱性条件下催化的 As(III) 氧化
摘要 黄铁矿是一种分布广泛的矿物,在自然环境中通过溶解氧 (DO) 催化 As(III) 氧化为 As(V);然而,黄铁矿在碱性条件下的催化机理尚不清楚。在这项研究中,我们观察到在碱性条件 (7.5-11.0) 下,随着 pH 值的增加,可溶性 As(III) 的氧化速率增加。ESR 分析和淬火实验排除了 •OH、O2•- 和 1O2 对 As(III) 氧化的贡献,并表明 Fe(III)-OOH 和/或 H2O2 在 pH 值为 9.0 和 11.0 时确实是主要的氧化物质,并且Fe(IV)=O 在 pH 值为 11.0 时也有贡献。一系列有机底物选择性氧化为相应的氧化产物证实了在碱性溶液或有机溶剂中黄铁矿体系中存在 Fe(III)-OOH 和/或 Fe(IV)=O。尽管在 pH 值为 9.0 和 11.0 时,葡萄糖的加入明显增加了 H2O2 的产生,但这种增加并没有明显加快 As(III) 的氧化速率,这意味着 H2O2 不是 As(III) 氧化的主要物质。这项研究为碱性条件下黄铁矿-溶解氧系统中的氧化物质提供了新的见解。
更新日期:2020-04-01
中文翻译:

Hydroperoxo [Fe(III)-OOH] 和ferryl-oxo [Fe(IV)=O] 氧化物质参与黄铁矿在碱性条件下催化的 As(III) 氧化
摘要 黄铁矿是一种分布广泛的矿物,在自然环境中通过溶解氧 (DO) 催化 As(III) 氧化为 As(V);然而,黄铁矿在碱性条件下的催化机理尚不清楚。在这项研究中,我们观察到在碱性条件 (7.5-11.0) 下,随着 pH 值的增加,可溶性 As(III) 的氧化速率增加。ESR 分析和淬火实验排除了 •OH、O2•- 和 1O2 对 As(III) 氧化的贡献,并表明 Fe(III)-OOH 和/或 H2O2 在 pH 值为 9.0 和 11.0 时确实是主要的氧化物质,并且Fe(IV)=O 在 pH 值为 11.0 时也有贡献。一系列有机底物选择性氧化为相应的氧化产物证实了在碱性溶液或有机溶剂中黄铁矿体系中存在 Fe(III)-OOH 和/或 Fe(IV)=O。尽管在 pH 值为 9.0 和 11.0 时,葡萄糖的加入明显增加了 H2O2 的产生,但这种增加并没有明显加快 As(III) 的氧化速率,这意味着 H2O2 不是 As(III) 氧化的主要物质。这项研究为碱性条件下黄铁矿-溶解氧系统中的氧化物质提供了新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号