Tetrahedron ( IF 2.1 ) Pub Date : 2020-01-25 , DOI: 10.1016/j.tet.2020.130979 Michael G. Siskos , Panayiotis C. Varras , Ioannis P. Gerothanassis
|
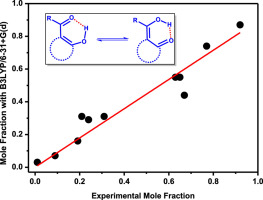
An approach for investigating the impacts of hydrogen bonding and stereochemical interactions of enol-enol tautomeric equilibria of β-dicarbonyl compounds is presented. DFT quantum chemical calculations of O–H⋯O 1H NMR chemical shifts of enol pairs, weighted by their proportions.as predicted by the relative free energies computed for them at the DFT level, agree well with experimental NMR chemical shifts. The main parameters affecting equilibrium constants for a set of eleven compounds, each one of which exists in two tautomeric forms in CHCl3 solution, are ring strain and steric repulsion of substituents with differences in O–H⋯O hydrogen bond lengths playing a secondary role.
中文翻译:

在研究烯醇-烯醇互变异构体平衡中的O-H⋯O 1 H NMR化学位移的DFT计算:探讨分子内氢键与立体电子相互作用的影响
提出了研究β-二羰基化合物的烯键-烯醇互变异构平衡的氢键和立体化学相互作用的影响的方法。烯醇对的O–H⋯O 1 H NMR化学位移的DFT量子化学计算,按其比例加权。如通过在DFT水平上为其计算的相对自由能所预测的,与实验NMR化学位移非常吻合。影响一组11种化合物的平衡常数的主要参数,其中每种化合物在CHCl 3溶液中以两种互变异构形式存在,其环应变和取代基的空间斥力(具有不同的O–H⋯O氢键长度)起着次要作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号