当前位置:
X-MOL 学术
›
Sci. Total Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lithium and strontium isotope dynamics in a carbonate island aquifer, Rottnest Island, Western Australia.
Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.scitotenv.2020.136906 A N Martin 1 , K Meredith 2 , M D Norman 3 , E Bryan 4 , A Baker 5
Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.scitotenv.2020.136906 A N Martin 1 , K Meredith 2 , M D Norman 3 , E Bryan 4 , A Baker 5
Affiliation
|
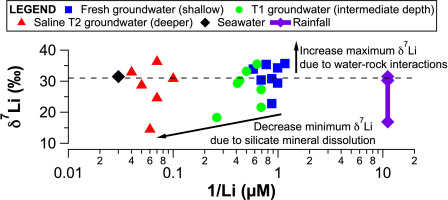
Water-rock interactions in aquifer systems are a key control on water quality but remain poorly understood. Lithium (Li) isotopes are useful for understanding water-rock interactions, but there are few data available for groundwater aquifers. Here we present a Li isotope dataset for rainfall and groundwater samples from a carbonate island aquifer system: Rottnest Island, Western Australia. This dataset was complemented by strontium (Sr) isotope and major and trace element data for groundwaters, and leaching experiments on bedrock samples. The δ7Li values and 87Sr/86Sr ratios of fresh groundwaters ranged from +23 to +36‰ and 0.709167 to 0.709198, respectively. Mass balance calculations indicated that silicate weathering supplied ~60 and 70% of dissolved Li and Sr in fresh groundwaters, respectively, with the remainder provided by atmospheric input, and carbonate weathering; for major cations, the majority of calcium and sodium (Na) are supplied by carbonate weathering and atmospheric input, respectively. The estimated low proportion of Sr produced by carbonate weathering was surprising in a carbonate aquifer, and the 87Sr/86Sr data indicated that the silicate Sr source had low Rb/Sr and 87Sr/86Sr ratios. There was an increase in the maximum δ7Li values in fresh groundwaters (+36‰) relative to the maximum value in rainfall and seawater (ca. +31‰). As clay minerals are undersaturated in fresh groundwaters, this increase may be explained by Li isotope fractionation associated with ion-exchange reactions on clays and iron(oxy)hydroxides. In the more saline groundwaters, the minimum δ7Li values decreased with depth to +14.5‰, suggesting increased silicate mineral dissolution in the deeper aquifer. These results reveal the importance of water-rock interactions in a coastal carbonate aquifer, and demonstrate the usefulness of Li isotopes for tracing weathering reactions in an environmental setting where traditional weathering tracers, such as sodium and Sr isotopes, are less appropriate.
中文翻译:

西澳大利亚州罗特尼斯岛碳酸盐岛含水层中的锂和锶同位素动力学。
含水层系统中的水-岩相互作用是控制水质的关键,但人们对此知之甚少。锂(Li)同位素对于理解水-岩相互作用非常有用,但很少有可用于地下水含水层的数据。在这里,我们展示了一个碳酸盐岛含水层系统(西澳大利亚罗特尼斯岛)的降雨和地下水样品的锂同位素数据集。该数据集补充了锶(Sr)同位素,地下水的主要和微量元素数据以及基岩样品的浸出实验。淡水的δ7Li值和87Sr / 86Sr之比分别为+23至+ 36‰和0.709167至0.709198。质量平衡计算表明,硅酸盐风化分别提供了淡水中约60%和70%的溶解的Li和Sr,其余的由大气输入提供,碳酸盐风化;对于主要阳离子,大部分钙和钠(Na)分别由碳酸盐风化作用和大气输入提供。在碳酸盐含水层中,碳酸盐风化产生的Sr的估计低比例是令人惊讶的,并且87Sr / 86Sr数据表明,硅酸盐Sr源的Rb / Sr和87Sr / 86Sr比例低。相对于降雨和海水的最大值(约+ 31‰),淡水的最大δ7Li值(+ 36‰)有所增加。由于粘土矿物质在新鲜地下水中的饱和度较低,因此这种增加可以用与在粘土和氢氧化铁(氧)上进行离子交换反应有关的锂同位素分馏来解释。在含盐量较高的地下水中,最小δ7Li值随深度降低至+ 14.5‰,表明在较深的含水层中硅酸盐矿物溶解增加。这些结果揭示了沿海碳酸盐岩含水层中水-岩相互作用的重要性,并证明了锂同位素在追踪不太适合使用传统风化示踪剂(例如钠和Sr同位素)的环境中追踪风化反应的有用性。
更新日期:2020-01-24
中文翻译:

西澳大利亚州罗特尼斯岛碳酸盐岛含水层中的锂和锶同位素动力学。
含水层系统中的水-岩相互作用是控制水质的关键,但人们对此知之甚少。锂(Li)同位素对于理解水-岩相互作用非常有用,但很少有可用于地下水含水层的数据。在这里,我们展示了一个碳酸盐岛含水层系统(西澳大利亚罗特尼斯岛)的降雨和地下水样品的锂同位素数据集。该数据集补充了锶(Sr)同位素,地下水的主要和微量元素数据以及基岩样品的浸出实验。淡水的δ7Li值和87Sr / 86Sr之比分别为+23至+ 36‰和0.709167至0.709198。质量平衡计算表明,硅酸盐风化分别提供了淡水中约60%和70%的溶解的Li和Sr,其余的由大气输入提供,碳酸盐风化;对于主要阳离子,大部分钙和钠(Na)分别由碳酸盐风化作用和大气输入提供。在碳酸盐含水层中,碳酸盐风化产生的Sr的估计低比例是令人惊讶的,并且87Sr / 86Sr数据表明,硅酸盐Sr源的Rb / Sr和87Sr / 86Sr比例低。相对于降雨和海水的最大值(约+ 31‰),淡水的最大δ7Li值(+ 36‰)有所增加。由于粘土矿物质在新鲜地下水中的饱和度较低,因此这种增加可以用与在粘土和氢氧化铁(氧)上进行离子交换反应有关的锂同位素分馏来解释。在含盐量较高的地下水中,最小δ7Li值随深度降低至+ 14.5‰,表明在较深的含水层中硅酸盐矿物溶解增加。这些结果揭示了沿海碳酸盐岩含水层中水-岩相互作用的重要性,并证明了锂同位素在追踪不太适合使用传统风化示踪剂(例如钠和Sr同位素)的环境中追踪风化反应的有用性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号