当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing the Signaling of GPCRs via Orthosteric Ions.
ACS Central Science ( IF 12.7 ) Pub Date : 2020-01-23 , DOI: 10.1021/acscentsci.9b01247 H C Stephen Chan 1 , Yueming Xu 2 , Liang Tan 2 , Horst Vogel 1, 3 , Jianjun Cheng 2 , Dong Wu 2 , Shuguang Yuan 1
ACS Central Science ( IF 12.7 ) Pub Date : 2020-01-23 , DOI: 10.1021/acscentsci.9b01247 H C Stephen Chan 1 , Yueming Xu 2 , Liang Tan 2 , Horst Vogel 1, 3 , Jianjun Cheng 2 , Dong Wu 2 , Shuguang Yuan 1
Affiliation
|
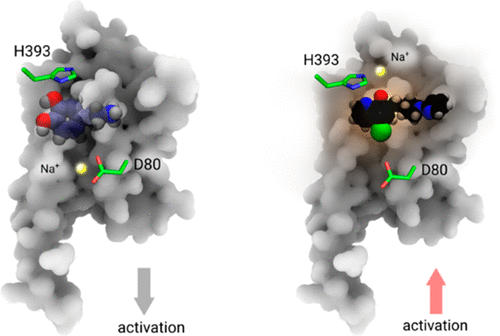
G protein-coupled receptors play essential roles in cellular processes such as neuronal signaling, vision, olfaction, tasting, and metabolism. As GPCRs are the most important drug targets, understanding their interactions with ligands is of utmost importance for discovering related new medicines. In many GPCRs, an allosteric sodium ion next to the highly conserved residue D2.50 has been proposed to stabilize the inactive receptor state by mediating interactions between transmembrane helices. Here, we probed the existence of internal and functionally important sodium ions in the dopamine D2 receptor, using molecular dynamics simulations. Besides a new sodium ion at the allosteric ligand binding site, we discovered an additional sodium ion, located close to the orthosteric ligand binding site. Through cell-based activation assays, the signaling of D2 receptor with site-specific mutations was tested against a series of chemically modified agonists. We concluded an important structural role of this newly discovered orthosteric sodium ion in modulating the receptor signaling: It enables the coordination of a polar residue in the ligand binding site with an appropriately designed agonist molecule. An identical interaction was also observed in a recently released high-resolution crystal structure of mu-opioid receptor, which was reresolved in this work. Probably because of similar interactions, various metal ions have been found to increase the signaling of many other GPCRs. This unique principle and strategy could be used to optimize the drug activity of GPCR. Our findings open a new mechanistic opportunity of GPCR signaling and help design the next generation of drugs targeting GPCRs.
中文翻译:

通过正构离子增强GPCR的信号传导。
G蛋白偶联受体在诸如神经元信号传导,视觉,嗅觉,品尝和代谢等细胞过程中起着至关重要的作用。由于GPCR是最重要的药物靶标,因此了解其与配体的相互作用对于发现相关的新药至关重要。在许多GPCR中,已经提出了高度保守残基D2.50旁的变构钠离子可通过介导跨膜螺旋之间的相互作用来稳定非活性受体状态。在这里,我们使用分子动力学模拟研究了多巴胺D2受体内部和功能上重要的钠离子的存在。除了在变构配体结合位点上有一个新的钠离子外,我们还发现了另一个靠近正构配体结合位点的钠离子。通过基于细胞的激活测定,针对一系列化学修饰的激动剂测试了具有位点特异性突变的D2受体的信号传导。我们总结了这种新发现的正构钠离子在调节受体信号传导中的重要结构作用:它使配体结合位点中的极性残基能够与适当设计的激动剂分子配合。在最近发布的μ阿片受体的高分辨率晶体结构中也观察到了相同的相互作用,该结构在这项工作中得到了解决。可能是由于相似的相互作用,已发现各种金属离子会增加许多其他GPCR的信号传导。这种独特的原理和策略可用于优化GPCR的药物活性。
更新日期:2020-02-26
中文翻译:

通过正构离子增强GPCR的信号传导。
G蛋白偶联受体在诸如神经元信号传导,视觉,嗅觉,品尝和代谢等细胞过程中起着至关重要的作用。由于GPCR是最重要的药物靶标,因此了解其与配体的相互作用对于发现相关的新药至关重要。在许多GPCR中,已经提出了高度保守残基D2.50旁的变构钠离子可通过介导跨膜螺旋之间的相互作用来稳定非活性受体状态。在这里,我们使用分子动力学模拟研究了多巴胺D2受体内部和功能上重要的钠离子的存在。除了在变构配体结合位点上有一个新的钠离子外,我们还发现了另一个靠近正构配体结合位点的钠离子。通过基于细胞的激活测定,针对一系列化学修饰的激动剂测试了具有位点特异性突变的D2受体的信号传导。我们总结了这种新发现的正构钠离子在调节受体信号传导中的重要结构作用:它使配体结合位点中的极性残基能够与适当设计的激动剂分子配合。在最近发布的μ阿片受体的高分辨率晶体结构中也观察到了相同的相互作用,该结构在这项工作中得到了解决。可能是由于相似的相互作用,已发现各种金属离子会增加许多其他GPCR的信号传导。这种独特的原理和策略可用于优化GPCR的药物活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号