当前位置:
X-MOL 学术
›
J. Fluorine Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Improved enantioselective gram scale synthesis route to N-Fmoc-protected monofluoroethylglycine
Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.jfluchem.2020.109453 Jakob Leppkes , Thomas Hohmann , Beate Koksch
中文翻译:

改进的对映选择性克规模的合成路线以N -Fmoc保护的单氟乙基甘氨酸
更新日期:2020-01-24
Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.jfluchem.2020.109453 Jakob Leppkes , Thomas Hohmann , Beate Koksch
|
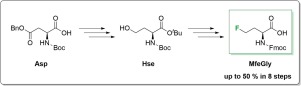
Fluorine, as a substituent in amino acids, has found its way into peptide and protein engineering. The basis for the use of this valuable tool is the synthetic accessibility of various fluorinated amino acids as building blocks of peptides and proteins. In this context, we present a straightforward eight-step synthesis of N-Fmoc-l-monofluoroethylglycine (MfeGly) via homoserine (Hse) as intermediate and using various nucleophilic fluorination strategies.
中文翻译:

改进的对映选择性克规模的合成路线以N -Fmoc保护的单氟乙基甘氨酸
氟作为氨基酸的取代基,已进入肽和蛋白质工程领域。使用这种有价值的工具的基础是各种氟化氨基酸作为肽和蛋白质构建单元的合成可及性。在这种情况下,我们提出的直接八步合成Ñ -Fmoc-升-monofluoroethylglycine(MfeGly)经由高丝氨酸(HSE)作为中间,以及使用各种亲核氟化策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号