Journal of Allergy and Clinical Immunology ( IF 14.2 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.jaci.2020.01.013 Milica M Grozdanovic 1 , Christine B Doyle 1 , Li Liu 1 , Brian T Maybruck 1 , Mark A Kwatia 1 , Nethaji Thiyagarajan 2 , K Ravi Acharya 2 , Steven J Ackerman 1
|
Background
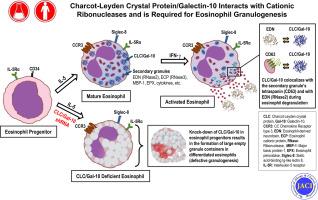
The human eosinophil Charcot-Leyden crystal (CLC) protein is a member of the Galectin superfamily and is also known as galectin-10 (Gal-10). CLC/Gal-10 forms the distinctive hexagonal bipyramidal crystals that are considered hallmarks of eosinophil participation in allergic responses and related inflammatory reactions; however, the glycan-containing ligands of CLC/Gal-10, its cellular function(s), and its role(s) in allergic diseases are unknown.
Objective
We sought to determine the binding partners of CLC/Gal-10 and elucidate its role in eosinophil biology.
Methods
Intracellular binding partners were determined by ligand blotting with CLC/Gal-10, followed by coimmunoprecipitation and coaffinity purifications. The role of CLC/Gal-10 in eosinophil function was determined by using enzyme activity assays, confocal microscopy, and short hairpin RNA knockout of CLC/Gal-10 expression in human CD34+ cord blood hematopoietic progenitors differentiated to eosinophils.
Results
CLC/Gal-10 interacts with both human eosinophil granule cationic ribonucleases (RNases), namely, eosinophil-derived neurotoxin (RNS2) and eosinophil cationic protein (RNS3), and with murine eosinophil-associated RNases. The interaction is independent of glycosylation and is not inhibitory toward endoRNase activity. Activation of eosinophils with INF-γ induces the rapid colocalization of CLC/Gal-10 with eosinophil-derived neurotoxin/RNS2 and CD63. Short hairpin RNA knockdown of CLC/Gal-10 in human cord blood–derived CD34+ progenitor cells impairs eosinophil granulogenesis.
Conclusions
CLC/Gal-10 functions as a carrier for the sequestration and vesicular transport of the potent eosinophil granule cationic RNases during both differentiation and degranulation, enabling their intracellular packaging and extracellular functions in allergic inflammation.
中文翻译:

Charcot-Leyden 晶体蛋白/半乳糖凝集素 10 与阳离子核糖核酸酶相互作用,是嗜酸性粒细胞形成所必需的。
背景
人嗜酸性粒细胞 Charcot-Leyden 晶体 (CLC) 蛋白是半乳凝素超家族的成员,也称为半乳凝素-10 (Gal-10)。CLC/Gal-10 形成独特的六角双锥体晶体,被认为是嗜酸性粒细胞参与过敏反应和相关炎症反应的标志;然而,CLC/Gal-10 的含聚糖配体、其细胞功能及其在过敏性疾病中的作用尚不清楚。
客观的
我们试图确定 CLC/Gal-10 的结合伙伴并阐明其在嗜酸性粒细胞生物学中的作用。
方法
通过用 CLC/Gal-10 进行配体印迹确定细胞内结合伙伴,然后进行免疫共沉淀和亲和纯化。CLC/Gal-10 在嗜酸性粒细胞功能中的作用是通过使用酶活性测定、共聚焦显微镜和短发夹 RNA 敲除人类 CD34 +脐血造血祖细胞分化为嗜酸性粒细胞中的 CLC/Gal-10 表达来确定的。
结果
CLC/Gal-10 与人嗜酸性粒细胞阳离子核糖核酸酶 (RNases),即嗜酸性粒细胞衍生的神经毒素 (RNS2) 和嗜酸性粒细胞阳离子蛋白 (RNS3),以及与鼠嗜酸性粒细胞相关的 RNases 相互作用。这种相互作用不依赖于糖基化,并且不会抑制内切核糖核酸酶的活性。用 INF-γ 激活嗜酸性粒细胞可诱导 CLC/Gal-10 与嗜酸性粒细胞衍生的神经毒素/RNS2 和 CD63 快速共定位。人脐血来源的 CD34 +祖细胞中 CLC/Gal-10 的短发夹 RNA 敲低会损害嗜酸性粒细胞的颗粒形成。
结论
CLC/Gal-10 在分化和脱粒过程中充当强效嗜酸性粒细胞颗粒阳离子 RNase 的螯合和囊泡转运载体,使其在过敏性炎症中发挥细胞内包装和细胞外功能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号