当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoinduced Production of Chlorine Molecules from Titanium Dioxide Surfaces Containing Chloride
Environmental Science & Technology Letters ( IF 10.9 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.estlett.9b00704 Yuanyuan Li 1, 2 , Wei Nie 1, 2 , Yuliang Liu 1, 2 , Dandan Huang 3 , Zheng Xu 1, 2 , Xiang Peng 4 , Christian George 5 , Chao Yan 6 , Yee Jun Tham 6 , Chuan Yu 4 , Men Xia 4 , Xiao Fu 4 , Xinfeng Wang 7 , Likun Xue 7 , Zhe Wang 8 , Zhengning Xu 1, 2 , Xuguang Chi 1, 2 , Tao Wang 4 , Aijun Ding 1, 2
Environmental Science & Technology Letters ( IF 10.9 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.estlett.9b00704 Yuanyuan Li 1, 2 , Wei Nie 1, 2 , Yuliang Liu 1, 2 , Dandan Huang 3 , Zheng Xu 1, 2 , Xiang Peng 4 , Christian George 5 , Chao Yan 6 , Yee Jun Tham 6 , Chuan Yu 4 , Men Xia 4 , Xiao Fu 4 , Xinfeng Wang 7 , Likun Xue 7 , Zhe Wang 8 , Zhengning Xu 1, 2 , Xuguang Chi 1, 2 , Tao Wang 4 , Aijun Ding 1, 2
Affiliation
|
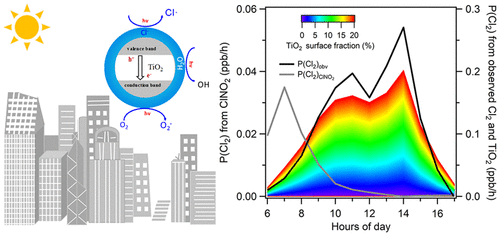
Titanium dioxide (TiO2) is extensively used with the process of urbanization and potentially influences atmospheric chemistry, which is yet unclear. In this work, we demonstrated strong production of Cl2 from illuminated KCl-coated TiO2 membranes and suggested an important daytime source of chlorine radicals. We found that water and oxygen were required for the reactions to proceed, and Cl2 production increased linearly with the amount of coated KCl, humidity of the carrier gas, and light intensity. These results suggested that water promotes the reactivity of coated KCl via interaction with the crystal lattice to release free chloride ions (Cl–). The free Cl– transfer charges to O2 via photoactivated TiO2 to form Cl2 and probably the O2– radical. In addition to Cl2, ClO and HOCl were also observed via the complex reactions between Cl/Cl2 and HOx. An intensive campaign was conducted in Shanghai, during which evident daytime peaks of Cl2 were observed. Estimated Cl2 production from TiO2 photocatalysis can be up to 0.2 ppb/h when the TiO2-containing surface reaches 20% of the urban surface, and highly correlated to the observed Cl2. Our results suggest a non-negligible role of TiO2 in atmospheric photochemistry via altering the radical budget.
中文翻译:

从含氯化物的二氧化钛表面光诱导生产氯分子
二氧化钛(TiO 2)在城市化过程中被广泛使用,并可能影响大气化学,目前尚不清楚。在这项工作中,我们证明了从照明的KCl涂层TiO 2膜可大量生产Cl 2,并提出了白天重要的氯自由基来源。我们发现,进行反应需要水和氧气,Cl 2的生成量随被覆KCl的量,载气的湿度和光强度线性增加。这些结果表明,水经由与晶格释放游离氯离子(CL相互作用促进涂布的KCl的反应性- )。免费的Cl –转移至O的费用2通过光活化的TiO 2,以形成氯2和可能是Ò 2 -基团。除了Cl 2以外,还通过Cl / Cl 2和HO x之间的复杂反应观察到ClO和HOCl 。在上海进行了一次密集运动,在此期间观察到明显的白天Cl 2高峰。当含TiO 2的表面达到城市表面的20%时,估计的TiO 2光催化生产的Cl 2可以高达0.2 ppb / h ,并且与观察到的Cl 2高度相关。我们的结果表明TiO 2的作用不可忽略 通过改变自由基的预算来进行大气光化学反应。
更新日期:2020-01-24
中文翻译:

从含氯化物的二氧化钛表面光诱导生产氯分子
二氧化钛(TiO 2)在城市化过程中被广泛使用,并可能影响大气化学,目前尚不清楚。在这项工作中,我们证明了从照明的KCl涂层TiO 2膜可大量生产Cl 2,并提出了白天重要的氯自由基来源。我们发现,进行反应需要水和氧气,Cl 2的生成量随被覆KCl的量,载气的湿度和光强度线性增加。这些结果表明,水经由与晶格释放游离氯离子(CL相互作用促进涂布的KCl的反应性- )。免费的Cl –转移至O的费用2通过光活化的TiO 2,以形成氯2和可能是Ò 2 -基团。除了Cl 2以外,还通过Cl / Cl 2和HO x之间的复杂反应观察到ClO和HOCl 。在上海进行了一次密集运动,在此期间观察到明显的白天Cl 2高峰。当含TiO 2的表面达到城市表面的20%时,估计的TiO 2光催化生产的Cl 2可以高达0.2 ppb / h ,并且与观察到的Cl 2高度相关。我们的结果表明TiO 2的作用不可忽略 通过改变自由基的预算来进行大气光化学反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号