当前位置:
X-MOL 学术
›
Colloids Surf. B Biointerfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of lactoferrin mesoporous silica nanoparticles for pemetrexed/ellagic acid synergistic breast cancer therapy.
Colloids and Surfaces B: Biointerfaces ( IF 5.8 ) Pub Date : 2020-01-24 , DOI: 10.1016/j.colsurfb.2020.110824 Omnia M Ali 1 , Adnan A Bekhit 2 , Sherine N Khattab 3 , Maged W Helmy 4 , Yasser S Abdel-Ghany 5 , Mohamed Teleb 6 , Ahmed O Elzoghby 7
Colloids and Surfaces B: Biointerfaces ( IF 5.8 ) Pub Date : 2020-01-24 , DOI: 10.1016/j.colsurfb.2020.110824 Omnia M Ali 1 , Adnan A Bekhit 2 , Sherine N Khattab 3 , Maged W Helmy 4 , Yasser S Abdel-Ghany 5 , Mohamed Teleb 6 , Ahmed O Elzoghby 7
Affiliation
|
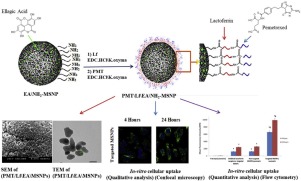
Despite the clinical approval of few nanomedicines for cancer therapy, some drawbacks still impede their improved efficiency including low drug loading, off-target toxicity and development of multi-drug resistance. Herein, lactoferrin (Lf)-coupled mesoporous silica nanoparticles (MSNPs) were developed for combined delivery of the cytotoxic drug pemetrexed (PMT) and the phytomedicine ellagic acid (EA) for synergistic breast cancer therapy. While the hydrophobic EA was physically encapsulated within the pores of MSNPs via the adsorptive properties of MSNPs and the electrostatic interactions between the negatively charged EA and positively charged amino modified MSNs, the highly water soluble PMT was chemically anchored to the Lf shell through chemical conjugation to the surface of lactoferrin coated MSNPs by carbodiimide reaction to avoid pre-mature drug release and systemic toxicity. The dual drug-loaded Lf-MSNPs (284 nm) demonstrated a sequential faster release of EA followed by a sustained release of PMT. The dual drug-loaded Lf-MSNPs exhibited highest cytotoxicity against MCF-7 (Michigan Cancer Foundation-7) breast cancer cells as revealed by the lowest combination index (CI = 0.885) compared to free drugs. The combination index value (< 1) revealed synergy between both loaded drugs. Furthermore, high cellular uptake of the nanocarriers into MCF-7 breast cancer cells was observed via Lf-receptor mediated endocytosis. Altogether, the dual drug-loaded Lf-targeted MSNPs showed to be a promising carrier for breast cancer therapy through triggering different signaling pathways, and hence overcoming the multi-drug resistance and minimizing the systemic toxicity.
中文翻译:

乳铁蛋白介孔二氧化硅纳米粒子的合成用于培美曲塞/鞣花酸协同乳腺癌治疗。
尽管很少有纳米药物在临床上用于癌症治疗,但仍有一些缺点仍然阻碍了其效率的提高,包括低载药量,脱靶毒性和多药耐药性的发展。在本文中,乳铁蛋白(Lf)偶联的介孔二氧化硅纳米颗粒(MSNPs)被开发用于联合递送培美曲塞(PMT)和植物新药鞣花酸(EA)的细胞毒性药物,用于协同性乳腺癌治疗。通过疏水性EA通过MSNP的吸附特性以及带负电的EA和带正电的氨基修饰的MSN之间的静电相互作用,将其物理包裹在MSNP的孔中,高度水溶性的PMT通过碳二亚胺反应通过化学偶联至乳铁蛋白包被的MSNPs表面而化学锚定在Lf壳上,以避免过早释放药物和全身毒性。载有双重药物的Lf-MSNP(284 nm)证明了EA依次快速释放,随后PMT持续释放。载有双重药物的Lf-MSNP与游离药物相比,对MCF-7(密歇根癌症基金会-7)乳腺癌细胞表现出最高的细胞毒性,这是由最低的组合指数(CI = 0.885)所揭示的。组合指数值(<1)显示了两种药物之间的协同作用。此外,通过Lf受体介导的内吞作用观察到纳米载体对MCF-7乳腺癌细胞的高细胞摄取。共,
更新日期:2020-01-24
中文翻译:

乳铁蛋白介孔二氧化硅纳米粒子的合成用于培美曲塞/鞣花酸协同乳腺癌治疗。
尽管很少有纳米药物在临床上用于癌症治疗,但仍有一些缺点仍然阻碍了其效率的提高,包括低载药量,脱靶毒性和多药耐药性的发展。在本文中,乳铁蛋白(Lf)偶联的介孔二氧化硅纳米颗粒(MSNPs)被开发用于联合递送培美曲塞(PMT)和植物新药鞣花酸(EA)的细胞毒性药物,用于协同性乳腺癌治疗。通过疏水性EA通过MSNP的吸附特性以及带负电的EA和带正电的氨基修饰的MSN之间的静电相互作用,将其物理包裹在MSNP的孔中,高度水溶性的PMT通过碳二亚胺反应通过化学偶联至乳铁蛋白包被的MSNPs表面而化学锚定在Lf壳上,以避免过早释放药物和全身毒性。载有双重药物的Lf-MSNP(284 nm)证明了EA依次快速释放,随后PMT持续释放。载有双重药物的Lf-MSNP与游离药物相比,对MCF-7(密歇根癌症基金会-7)乳腺癌细胞表现出最高的细胞毒性,这是由最低的组合指数(CI = 0.885)所揭示的。组合指数值(<1)显示了两种药物之间的协同作用。此外,通过Lf受体介导的内吞作用观察到纳米载体对MCF-7乳腺癌细胞的高细胞摄取。共,



























 京公网安备 11010802027423号
京公网安备 11010802027423号