当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Disruption of the HIV-1 Envelope allosteric network blocks CD4-induced rearrangements.
Nature Communications ( IF 16.6 ) Pub Date : 2020-01-24 , DOI: 10.1038/s41467-019-14196-w Rory Henderson 1, 2 , Maolin Lu 3 , Ye Zhou 4 , Zekun Mu 5 , Robert Parks 2 , Qifeng Han 1, 2 , Allen L Hsu 6 , Elizabeth Carter 1, 2 , Scott C Blanchard 7, 8 , R J Edwards 1, 2 , Kevin Wiehe 1, 2 , Kevin O Saunders 2, 9 , Mario J Borgnia 6 , Alberto Bartesaghi 4, 10, 11 , Walther Mothes 3 , Barton F Haynes 1, 2, 5 , Priyamvada Acharya 2, 9 , S Munir Alam 1, 2, 12
Nature Communications ( IF 16.6 ) Pub Date : 2020-01-24 , DOI: 10.1038/s41467-019-14196-w Rory Henderson 1, 2 , Maolin Lu 3 , Ye Zhou 4 , Zekun Mu 5 , Robert Parks 2 , Qifeng Han 1, 2 , Allen L Hsu 6 , Elizabeth Carter 1, 2 , Scott C Blanchard 7, 8 , R J Edwards 1, 2 , Kevin Wiehe 1, 2 , Kevin O Saunders 2, 9 , Mario J Borgnia 6 , Alberto Bartesaghi 4, 10, 11 , Walther Mothes 3 , Barton F Haynes 1, 2, 5 , Priyamvada Acharya 2, 9 , S Munir Alam 1, 2, 12
Affiliation
|
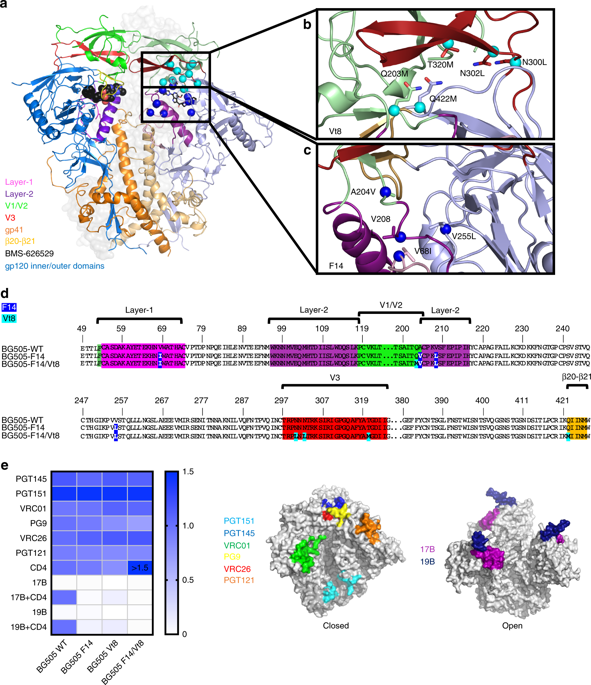
The trimeric HIV-1 Envelope protein (Env) mediates viral-host cell fusion via a network of conformational transitions, with allosteric elements in each protomer orchestrating host receptor-induced exposure of the co-receptor binding site and fusion elements. To understand the molecular details of this allostery, here, we introduce Env mutations aimed to prevent CD4-induced rearrangements in the HIV-1 BG505 Env trimer. Binding analysis and single-molecule Förster Resonance Energy Transfer confirm that these mutations prevent CD4-induced transitions of the HIV-1 Env. Structural analysis by single-particle cryo-electron microscopy performed on the BG505 SOSIP mutant Env proteins shows rearrangements in the gp120 topological layer contacts with gp41. Displacement of a conserved tryptophan (W571) from its typical pocket in these Env mutants renders the Env insensitive to CD4 binding. These results reveal the critical function of W571 as a conformational switch in Env allostery and receptor-mediated viral entry and provide insights on Env conformation that are relevant for vaccine design.
中文翻译:

HIV-1 包膜变构网络的破坏会阻止 CD4 诱导的重排。
三聚体 HIV-1 包膜蛋白 (Env) 通过构象转变网络介导病毒-宿主细胞融合,每个原聚体中的变构元件协调宿主受体诱导的共受体结合位点和融合元件的暴露。为了了解这种变构的分子细节,在这里,我们引入了 Env 突变,旨在防止 HIV-1 BG505 Env 三聚体中 CD4 诱导的重排。结合分析和单分子福斯特共振能量转移证实,这些突变可以阻止 CD4 诱导的 HIV-1 包膜转变。通过单粒子冷冻电子显微镜对 BG505 SOSIP 突变体 Env 蛋白进行的结构分析显示 gp120 拓扑层与 gp41 接触的重排。在这些 Env 突变体中,保守色氨酸 (W571) 从其典型口袋中被置换,使得 Env 对 CD4 结合不敏感。这些结果揭示了 W571 作为 Env 变构和受体介导的病毒进入中构象开关的关键功能,并提供了与疫苗设计相关的 Env 构象的见解。
更新日期:2020-01-24
中文翻译:

HIV-1 包膜变构网络的破坏会阻止 CD4 诱导的重排。
三聚体 HIV-1 包膜蛋白 (Env) 通过构象转变网络介导病毒-宿主细胞融合,每个原聚体中的变构元件协调宿主受体诱导的共受体结合位点和融合元件的暴露。为了了解这种变构的分子细节,在这里,我们引入了 Env 突变,旨在防止 HIV-1 BG505 Env 三聚体中 CD4 诱导的重排。结合分析和单分子福斯特共振能量转移证实,这些突变可以阻止 CD4 诱导的 HIV-1 包膜转变。通过单粒子冷冻电子显微镜对 BG505 SOSIP 突变体 Env 蛋白进行的结构分析显示 gp120 拓扑层与 gp41 接触的重排。在这些 Env 突变体中,保守色氨酸 (W571) 从其典型口袋中被置换,使得 Env 对 CD4 结合不敏感。这些结果揭示了 W571 作为 Env 变构和受体介导的病毒进入中构象开关的关键功能,并提供了与疫苗设计相关的 Env 构象的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号