当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Mizoroki‐Heck Reaction with Internal Olefins: Reactivities and Stereoselectivities
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-02-14 , DOI: 10.1002/ajoc.201900741 Yusei Nakashima 1 , Goki Hirata 1 , Tom D. Sheppard 2 , Takashi Nishikata 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-02-14 , DOI: 10.1002/ajoc.201900741 Yusei Nakashima 1 , Goki Hirata 1 , Tom D. Sheppard 2 , Takashi Nishikata 1
Affiliation
|
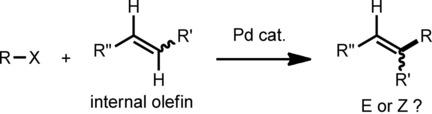
The Mizoroki‐Heck reaction is one of the most valuable reactions for functionalizing C−C double bonds in the presence of a Pd catalyst. This protocol is suitable for the reaction of a C(sp2)‐halide with a terminal olefin to produce a trans‐1,2‐disubstituted olefin. However, reports of the Mizoroki‐Heck reaction of internal olefins are rare and impractical due to the low reactivity of internal olefins and problems of product diastereoselectivity. In this review, we summarize Mizoroki‐Heck reactions of internal olefins with aryl or alkyl halides to illustrate their reactivities and stereoselectivities.
中文翻译:

与内部烯烃的Mizoroki-Heck反应:反应性和立体选择性
Mizoroki-Heck反应是在Pd催化剂存在下官能化C-C双键的最有价值的反应之一。该方案适用于C(sp 2)-卤化物与末端烯烃的反应,以生成反式1,2-二取代的烯烃。然而,由于内烯烃的低反应性和产物非对映选择性的问题,关于内烯烃的Mizoroki-Heck反应的报道很少,不切实际。在这篇综述中,我们总结了内烯烃与芳基或烷基卤化物的Mizoroki-Heck反应,以说明它们的反应性和立体选择性。
更新日期:2020-04-21
中文翻译:

与内部烯烃的Mizoroki-Heck反应:反应性和立体选择性
Mizoroki-Heck反应是在Pd催化剂存在下官能化C-C双键的最有价值的反应之一。该方案适用于C(sp 2)-卤化物与末端烯烃的反应,以生成反式1,2-二取代的烯烃。然而,由于内烯烃的低反应性和产物非对映选择性的问题,关于内烯烃的Mizoroki-Heck反应的报道很少,不切实际。在这篇综述中,我们总结了内烯烃与芳基或烷基卤化物的Mizoroki-Heck反应,以说明它们的反应性和立体选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号