当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Studies on Lewis‐Acid Induced Reactions of 8‐Methoxy[2.2]metacyclophanes: A New Synthetic Route to Alkylated Pyrenes
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-01-24 , DOI: 10.1002/slct.201903048 Md. Monarul Islam 1, 2, 3 , Xing Feng 3 , Chuan‐Zeng Wang 2 , Shofiur Rahman 4, 5 , Abdullah Alodhayb 4, 6 , Paris E. Georghiou 5 , Taisuke Matsumoto 7 , Junji Tanaka 7 , Carl Redshaw 8 , Takehiko Yamato 2, 9
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-01-24 , DOI: 10.1002/slct.201903048 Md. Monarul Islam 1, 2, 3 , Xing Feng 3 , Chuan‐Zeng Wang 2 , Shofiur Rahman 4, 5 , Abdullah Alodhayb 4, 6 , Paris E. Georghiou 5 , Taisuke Matsumoto 7 , Junji Tanaka 7 , Carl Redshaw 8 , Takehiko Yamato 2, 9
Affiliation
|
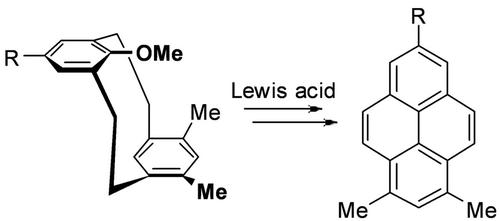
Anti‐8‐methoxy[2.2]metacyclophanes (MCPs) 5 a–b were obtained via pyrolysis of the corresponding syn‐thiatetraoxide cyclophanes 4 a–b. Coupling reactions of 4‐tert‐butyl‐1‐methoxy‐2,6‐bis(mercaptomethyl)benzenes 1 a–b and 1,5‐bis(chloro‐methyl)‐2,4‐dimethylbenzene 2 under high dilution conditions afforded only the syn‐conformers of 9‐methoxy‐2,11‐dithia[3.3]metacyclophanes 3 a–b, which with m‐CPBA formed the corresponding syn‐tetraoxides 4 a–b. Lewis acid (TICl4/AlCl3‐MeNO2) or iodine‐catalyzed reactions of 5 b under various conditions led to transannular cyclization to afford tetrahydropyrene 6 b and pyrene derivative 7 b and/or de‐tert‐butylated 6 a. Iodine‐catalyzed reaction of 5 a afforded tetrahydropyrene 6 a. These findings suggest the potential for a new route to alkylated pyrenes via strained and alkylated metacyclophanes. Density functional theory (DFT) studies were carried out to investigate the conformational characteristics of 3–5.
中文翻译:

刘易斯酸诱导的8-甲氧基[2.2]亚甲基环烷反应的研究:一种合成烷基化比利牛斯的新途径
反-8-甲氧基[2.2] metacyclophanes(MCP)的5 一- b分别经由相应的热解获得的顺式-thiatetraoxide环芳4 一- b。偶合4-的反应叔丁基-1-甲氧基-2,6-双(巯基甲基)苯1 一- b和1,5-双(氯甲基)-2,4-二甲基苯2高度稀释的条件下仅得到所述顺式-9-甲氧基-2,11-二硫杂的-conformers [3.3] metacyclophanes 3 一- b,其与米-CPBA形成的相应顺式-四 氧化物4 a – b。路易斯酸(的TiCl 4 /的AlCl 3 -MeNO 2)或碘催化的反应5 b导致跨环环化在各种条件下,得到四氢芘6 b和芘衍生物7 b和/或脱叔-butylated 6 一个。碘催化5 a生成四氢py 6 a。这些发现暗示了通过应变和烷基化的亚甲基环烷烃通往烷基化pyr的新途径的潜力。进行了密度泛函理论(DFT)研究以研究3–5的构象特征。
更新日期:2020-01-24
中文翻译:

刘易斯酸诱导的8-甲氧基[2.2]亚甲基环烷反应的研究:一种合成烷基化比利牛斯的新途径
反-8-甲氧基[2.2] metacyclophanes(MCP)的5 一- b分别经由相应的热解获得的顺式-thiatetraoxide环芳4 一- b。偶合4-的反应叔丁基-1-甲氧基-2,6-双(巯基甲基)苯1 一- b和1,5-双(氯甲基)-2,4-二甲基苯2高度稀释的条件下仅得到所述顺式-9-甲氧基-2,11-二硫杂的-conformers [3.3] metacyclophanes 3 一- b,其与米-CPBA形成的相应顺式-四 氧化物4 a – b。路易斯酸(的TiCl 4 /的AlCl 3 -MeNO 2)或碘催化的反应5 b导致跨环环化在各种条件下,得到四氢芘6 b和芘衍生物7 b和/或脱叔-butylated 6 一个。碘催化5 a生成四氢py 6 a。这些发现暗示了通过应变和烷基化的亚甲基环烷烃通往烷基化pyr的新途径的潜力。进行了密度泛函理论(DFT)研究以研究3–5的构象特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号