Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-01-24 , DOI: 10.1016/j.molliq.2020.112552 Yang Liu , Yonggang Yang , Xueli Jia , Qianfei Ma , Yuanyuan He , Hongsheng Zhai , Yingying Zhang , Yufang Liu
|
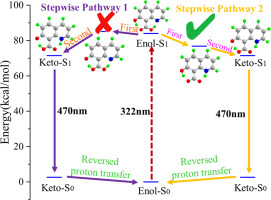
The excited-state molecular dynamics of 7-hydroxyquinoline-8-carboxylic acid (HCA) were studied to observe its fluorescent properties and proton transfer process. The two intramolecular hydrogen bonds (O-H⋯O and O-H⋯N) of the Enol form were both strengthened after photoexcitation to the first excited (S1) state. The experimentally observed fluorescence emission (465 nm) was attributed to the theoretical Keto form (470 nm), which implies the occurrence of an excited-state intramolecular double proton transfer (ESIDPT) process. The nonadiabatic dynamics results demonstrate that a single proton transfer from the carboxylic to the nitrogen atom (process-A) occurs in 55 fs, which excludes the ESIDPT concert pathway. The potential energy surface results indicate that process-A (0.08 kcal/mol) induces the occurrence of a second proton transfer from the phenol to the oxygen atom (process-B). Compared to the stepwise ESIDPT reaction that begins with process-B (1.53 kcal/mol), process-A is energy favorable in the S1 state. Therefore, we propose a reaction path of the following: Enol in the ground state (S0) → Enol in the S1 state → proton transferred Keto in the S1 state (stepwise pathway begins with process-A) → Keto in the S0 state (fluorescence emission at 470 nm) → Enol in the S0 state (reversed proton transfer). This process confirms what was predicted by Chou (J. Phys. Chem. Lett. 2011, 2, 3063).
中文翻译:

7-羟基喹啉-8-羧酸的激发态分子内双质子转移和光谱行为的理论研究
研究了7-羟基喹啉-8-羧酸(HCA)的激发态分子动力学,观察其荧光性质和质子转移过程。在光激发至第一次激发后,烯醇形式的两个分子内氢键(OH⋯O和OH⋯N)均被增强(S 1)状态。实验观察到的荧光发射(465 nm)归因于理论上的酮基形式(470 nm),这意味着发生了激发态分子内双质子转移(ESIDPT)过程。非绝热动力学结果表明,从羧基到氮原子的单质子转移在55 fs内发生,这不包括ESIDPT协同途径。势能表面结果表明,过程A(0.08 kcal / mol)引发了第二次质子从酚到氧原子的转移(过程B)。与从过程B开始的逐步ESIDPT反应(1.53 kcal / mol)相比,过程A在S 1状态下能量有利。因此,我们提出以下反应路径:基态的烯醇(S 0)→处于S 1状态的烯醇→质子转移到处于S 1状态的酮基(逐步途径始于过程A)→处于S 0状态的酮基(在470 nm处发出荧光)→处于S 0状态的烯醇(反转质子)转让)。该过程证实了Chou(J. Phys。Chem。Lett。2011,2,3063)的预测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号