Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial.
The Lancet ( IF 98.4 ) Pub Date : 2020-01-25 , DOI: 10.1016/s0140-6736(19)32973-3 Matthew K Hoffman 1 , Shivaprasad S Goudar 2 , Bhalachandra S Kodkany 2 , Mrityunjay Metgud 2 , Manjunath Somannavar 2 , Jean Okitawutshu 3 , Adrien Lokangaka 3 , Antoinette Tshefu 3 , Carl L Bose 4 , Abigail Mwapule 5 , Musaku Mwenechanya 5 , Elwyn Chomba 5 , Waldemar A Carlo 6 , Javier Chicuy 7 , Lester Figueroa 7 , Ana Garces 7 , Nancy F Krebs 8 , Saleem Jessani 9 , Farnaz Zehra 9 , Sarah Saleem 9 , Robert L Goldenberg 10 , Kunal Kurhe 11 , Prabir Das 11 , Archana Patel 11 , Patricia L Hibberd 12 , Emmah Achieng 13 , Paul Nyongesa 13 , Fabian Esamai 13 , Edward A Liechty 14 , Norman Goco 15 , Jennifer Hemingway-Foday 15 , Janet Moore 15 , Tracy L Nolen 15 , Elizabeth M McClure 15 , Marion Koso-Thomas 16 , Menachem Miodovnik 16 , R Silver 17 , Richard J Derman 18 ,
The Lancet ( IF 98.4 ) Pub Date : 2020-01-25 , DOI: 10.1016/s0140-6736(19)32973-3 Matthew K Hoffman 1 , Shivaprasad S Goudar 2 , Bhalachandra S Kodkany 2 , Mrityunjay Metgud 2 , Manjunath Somannavar 2 , Jean Okitawutshu 3 , Adrien Lokangaka 3 , Antoinette Tshefu 3 , Carl L Bose 4 , Abigail Mwapule 5 , Musaku Mwenechanya 5 , Elwyn Chomba 5 , Waldemar A Carlo 6 , Javier Chicuy 7 , Lester Figueroa 7 , Ana Garces 7 , Nancy F Krebs 8 , Saleem Jessani 9 , Farnaz Zehra 9 , Sarah Saleem 9 , Robert L Goldenberg 10 , Kunal Kurhe 11 , Prabir Das 11 , Archana Patel 11 , Patricia L Hibberd 12 , Emmah Achieng 13 , Paul Nyongesa 13 , Fabian Esamai 13 , Edward A Liechty 14 , Norman Goco 15 , Jennifer Hemingway-Foday 15 , Janet Moore 15 , Tracy L Nolen 15 , Elizabeth M McClure 15 , Marion Koso-Thomas 16 , Menachem Miodovnik 16 , R Silver 17 , Richard J Derman 18 ,
Affiliation
|
BACKGROUND
Preterm birth remains a common cause of neonatal mortality, with a disproportionately high burden in low-income and middle-income countries. Meta-analyses of low-dose aspirin to prevent pre-eclampsia suggest that the incidence of preterm birth might also be decreased, particularly if initiated before 16 weeks of gestation.
METHODS
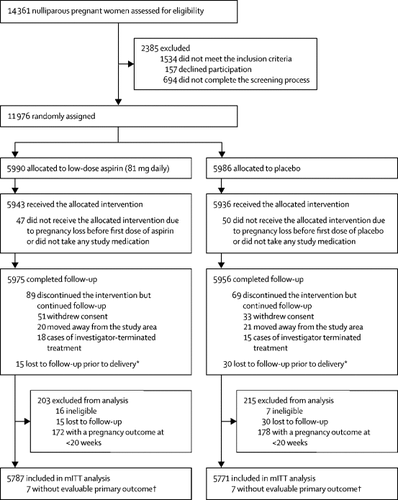
ASPIRIN was a randomised, multicountry, double-masked, placebo-controlled trial of low-dose aspirin (81 mg daily) initiated between 6 weeks and 0 days of pregnancy, and 13 weeks and 6 days of pregnancy, in nulliparous women with an ultrasound confirming gestational age and a singleton viable pregnancy. Participants were enrolled at seven community sites in six countries (two sites in India and one site each in the Democratic Republic of the Congo, Guatemala, Kenya, Pakistan, and Zambia). Participants were randomly assigned (1:1, stratified by site) to receive aspirin or placebo tablets of identical appearance, via a sequence generated centrally by the data coordinating centre at Research Triangle Institute International (Research Triangle Park, NC, USA). Treatment was masked to research staff, health providers, and patients, and continued until 36 weeks and 7 days of gestation or delivery. The primary outcome of incidence of preterm birth, defined as the number of deliveries before 37 weeks' gestational age, was analysed in randomly assigned women with pregnancy outcomes at or after 20 weeks, according to a modified intention-to-treat (mITT) protocol. Analyses of our binary primary outcome involved a Cochran-Mantel-Haenszel test stratified by site, and generalised linear models to obtain relative risk (RR) estimates and associated confidence intervals. Serious adverse events were assessed in all women who received at least one dose of drug or placebo. This study is registered with ClinicalTrials.gov, NCT02409680, and the Clinical Trial Registry-India, CTRI/2016/05/006970.
FINDINGS
From March 23, 2016 to June 30, 2018, 14 361 women were screened for inclusion and 11 976 women aged 14-40 years were randomly assigned to receive low-dose aspirin (5990 women) or placebo (5986 women). 5780 women in the aspirin group and 5764 in the placebo group were evaluable for the primary outcome. Preterm birth before 37 weeks occurred in 668 (11·6%) of the women who took aspirin and 754 (13·1%) of those who took placebo (RR 0·89 [95% CI 0·81 to 0·98], p=0·012). In women taking aspirin, we also observed significant reductions in perinatal mortality (0·86 [0·73-1·00], p=0·048), fetal loss (infant death after 16 weeks' gestation and before 7 days post partum; 0·86 [0·74-1·00], p=0·039), early preterm delivery (<34 weeks; 0·75 [0·61-0·93], p=0·039), and the incidence of women who delivered before 34 weeks with hypertensive disorders of pregnancy (0·38 [0·17-0·85], p=0·015). Other adverse maternal and neonatal events were similar between the two groups.
INTERPRETATION
In populations of nulliparous women with singleton pregnancies from low-income and middle-income countries, low-dose aspirin initiated between 6 weeks and 0 days of gestation and 13 weeks and 6 days of gestation resulted in a reduced incidence of preterm delivery before 37 weeks, and reduced perinatal mortality.
FUNDING
Eunice Kennedy Shriver National Institute of Child Health and Human Development.
中文翻译:

低剂量阿司匹林用于预防单胎妊娠未产妇早产(阿司匹林):一项随机、双盲、安慰剂对照试验。
背景技术早产仍然是新生儿死亡的常见原因,在低收入和中等收入国家中负担过高。使用低剂量阿司匹林预防先兆子痫的荟萃分析表明,早产的发生率也可能降低,特别是如果在妊娠 16 周之前开始服用的话。方法 阿司匹林是一项随机、多国、双盲、安慰剂对照试验,在妊娠 6 周至 0 天和妊娠 13 周至 6 天期间开始服用低剂量阿司匹林(每天 81 毫克),受试者为患有以下疾病的未产妇:超声波确认胎龄和单胎可行妊娠。参与者在六个国家的七个社区站点注册(印度两个站点,刚果民主共和国、危地马拉、肯尼亚、巴基斯坦和赞比亚各一个站点)。通过国际三角研究所(美国北卡罗来纳州三角研究园)数据协调中心集中生成的序列,参与者被随机分配(1:1,按地点分层)接受外观相同的阿司匹林或安慰剂药片。治疗对研究人员、医疗服务提供者和患者不公开,并持续至妊娠或分娩 36 周零 7 天。根据修改后的意向治疗 (mITT) 方案,对随机分配的 20 周或 20 周后妊娠结果的妇女进行了分析,即早产发生率的主要结果(定义为 37 周胎龄之前的分娩次数) 。我们对二元主要结果的分析涉及按地点分层的 Cochran-Mantel-Haenszel 检验和广义线性模型,以获得相对风险 (RR) 估计值和相关的置信区间。 对所有接受至少一剂药物或安慰剂的女性的严重不良事件进行了评估。本研究已在 ClinicalTrials.gov(NCT02409680)和印度临床试验注册中心(CTRI/2016/05/006970)注册。结果 从2016年3月23日到2018年6月30日,对14 361名女性进行了纳入筛选,11 976名14-40岁的女性被随机分配接受低剂量阿司匹林(5990名女性)或安慰剂(5986名女性)。阿司匹林组的 5780 名女性和安慰剂组的 5764 名女性接受了主要结局的评估。服用阿司匹林的妇女中有 668 名 (11·6%) 发生了 37 周前早产,服用安慰剂的妇女中有 754 名 (13·1%) 发生了 37 周前早产(RR 0·89 [95% CI 0·81 至 0·98] ,p=0·012)。在服用阿司匹林的女性中,我们还观察到围产期死亡率(0·86 [0·73-1·00],p=0·048)、胎儿流产(妊娠 16 周后和产后 7 天前的婴儿死亡)显着降低。 ;0·86 [0·74-1·00],p=0·039),早期早产(<34 周;0·75 [0·61-0·93],p=0·039),以及34 周前分娩的妇女患有妊娠期高血压疾病的发生率 (0·38 [0·17-0·85],p=0·015)。两组之间的其他不良孕产妇和新生儿事件相似。解释 在来自低收入和中等收入国家的单胎妊娠未产妇人群中,妊娠 6 周至 0 天和妊娠 13 周至 6 天开始服用低剂量阿司匹林可降低 37 岁之前的早产发生率。周,并降低围产期死亡率。资助尤尼斯·肯尼迪·施赖弗国家儿童健康和人类发展研究所。
更新日期:2020-01-24
中文翻译:

低剂量阿司匹林用于预防单胎妊娠未产妇早产(阿司匹林):一项随机、双盲、安慰剂对照试验。
背景技术早产仍然是新生儿死亡的常见原因,在低收入和中等收入国家中负担过高。使用低剂量阿司匹林预防先兆子痫的荟萃分析表明,早产的发生率也可能降低,特别是如果在妊娠 16 周之前开始服用的话。方法 阿司匹林是一项随机、多国、双盲、安慰剂对照试验,在妊娠 6 周至 0 天和妊娠 13 周至 6 天期间开始服用低剂量阿司匹林(每天 81 毫克),受试者为患有以下疾病的未产妇:超声波确认胎龄和单胎可行妊娠。参与者在六个国家的七个社区站点注册(印度两个站点,刚果民主共和国、危地马拉、肯尼亚、巴基斯坦和赞比亚各一个站点)。通过国际三角研究所(美国北卡罗来纳州三角研究园)数据协调中心集中生成的序列,参与者被随机分配(1:1,按地点分层)接受外观相同的阿司匹林或安慰剂药片。治疗对研究人员、医疗服务提供者和患者不公开,并持续至妊娠或分娩 36 周零 7 天。根据修改后的意向治疗 (mITT) 方案,对随机分配的 20 周或 20 周后妊娠结果的妇女进行了分析,即早产发生率的主要结果(定义为 37 周胎龄之前的分娩次数) 。我们对二元主要结果的分析涉及按地点分层的 Cochran-Mantel-Haenszel 检验和广义线性模型,以获得相对风险 (RR) 估计值和相关的置信区间。 对所有接受至少一剂药物或安慰剂的女性的严重不良事件进行了评估。本研究已在 ClinicalTrials.gov(NCT02409680)和印度临床试验注册中心(CTRI/2016/05/006970)注册。结果 从2016年3月23日到2018年6月30日,对14 361名女性进行了纳入筛选,11 976名14-40岁的女性被随机分配接受低剂量阿司匹林(5990名女性)或安慰剂(5986名女性)。阿司匹林组的 5780 名女性和安慰剂组的 5764 名女性接受了主要结局的评估。服用阿司匹林的妇女中有 668 名 (11·6%) 发生了 37 周前早产,服用安慰剂的妇女中有 754 名 (13·1%) 发生了 37 周前早产(RR 0·89 [95% CI 0·81 至 0·98] ,p=0·012)。在服用阿司匹林的女性中,我们还观察到围产期死亡率(0·86 [0·73-1·00],p=0·048)、胎儿流产(妊娠 16 周后和产后 7 天前的婴儿死亡)显着降低。 ;0·86 [0·74-1·00],p=0·039),早期早产(<34 周;0·75 [0·61-0·93],p=0·039),以及34 周前分娩的妇女患有妊娠期高血压疾病的发生率 (0·38 [0·17-0·85],p=0·015)。两组之间的其他不良孕产妇和新生儿事件相似。解释 在来自低收入和中等收入国家的单胎妊娠未产妇人群中,妊娠 6 周至 0 天和妊娠 13 周至 6 天开始服用低剂量阿司匹林可降低 37 岁之前的早产发生率。周,并降低围产期死亡率。资助尤尼斯·肯尼迪·施赖弗国家儿童健康和人类发展研究所。











































 京公网安备 11010802027423号
京公网安备 11010802027423号