Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): a randomised, double-blind, placebo-controlled trial.
The Lancet ( IF 98.4 ) Pub Date : 2020-01-25 , DOI: 10.1016/s0140-6736(19)32981-2 Andrew Lotery 1 , Sobha Sivaprasad 2 , Abby O'Connell 3 , Rosie A Harris 3 , Lucy Culliford 3 , Lucy Ellis 3 , Angela Cree 1 , Savita Madhusudhan 4 , Francine Behar-Cohen 5 , Usha Chakravarthy 6 , Tunde Peto 6 , Chris A Rogers 3 , Barnaby C Reeves 3 ,
The Lancet ( IF 98.4 ) Pub Date : 2020-01-25 , DOI: 10.1016/s0140-6736(19)32981-2 Andrew Lotery 1 , Sobha Sivaprasad 2 , Abby O'Connell 3 , Rosie A Harris 3 , Lucy Culliford 3 , Lucy Ellis 3 , Angela Cree 1 , Savita Madhusudhan 4 , Francine Behar-Cohen 5 , Usha Chakravarthy 6 , Tunde Peto 6 , Chris A Rogers 3 , Barnaby C Reeves 3 ,
Affiliation
|
BACKGROUND
In chronic central serous chorioretinopathy (CSCR), fluid accumulates in the subretinal space. CSCR is a common visually disabling condition that develops in individuals up to 60 years of age, and there is no definitive treatment. Previous research suggests the mineralocorticoid receptor antagonist, eplerenone, is effective for treating CSCR; however, this drug is not licensed for the treatment of patients with CSCR. We aimed to evaluate whether eplerenone was superior to placebo in terms of improving visual acuity in patients with chronic CSCR.
METHODS
This randomised, double-blind, parallel-group, multicentre placebo-controlled trial was done at 22 hospitals in the UK. Participants were eligible if they were aged 18-60 years and had had treatment-naive CSCR for 4 months or more. Patients were randomly assigned (1:1) to either the eplerenone or the placebo group by a trial statistician through a password-protected system online. Allocation was stratified by best-corrected visual acuity (BCVA) and hospital. Patients were given either oral eplerenone (25 mg/day for 1 week, increasing to 50 mg/day for up to 12 months) plus usual care or placebo plus usual care for up to 12 months. All participants, care teams, outcome assessors, pharmacists, and members of the trial management group were masked to the treatment allocation. The primary outcome was BCVA, measured as letters read, at 12 months. All outcomes apart from safety were analysed on a modified intention-to-treat basis (participants who withdrew consent without contributing a post-randomisation BCVA measurement were excluded from the primary analysis population and from most secondary analysis populations). The trial is registered with ISRCTN, ISRCTN92746680, and is completed.
FINDINGS
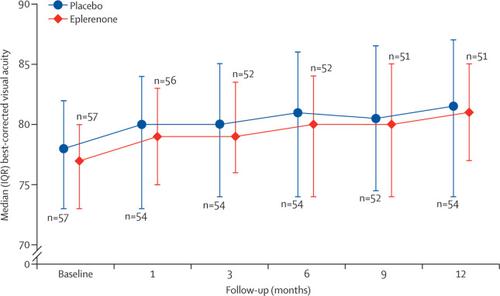
Between Jan 11, 2017, and Feb 22, 2018, we enrolled and randomly assigned 114 patients to receive either eplerenone (n=57) or placebo (n=57). Three participants in the placebo group withdrew consent without contributing a post-randomisation BCVA measurement and were excluded from the primary outcome analysis population. All patients from the eplerenone group and 54 patients from the placebo group were included in the primary outcome. Modelled mean BCVA at 12 months was 79·5 letters (SD 4·5) in the placebo group and 80·4 letters (4·6) in the eplerenone group, with an adjusted estimated mean difference of 1·73 letters (95% CI -1·12 to 4·57; p=0·24) at 12 months. Hyperkalaemia occurred in eight (14%) patients in each group. No serious adverse events were reported in the eplerenone group and three unrelated serious adverse events were reported in the placebo group (myocardial infarction [anticipated], diverticulitis [unanticipated], and metabolic surgery [unanticipated]).
INTERPRETATION
Eplerenone was not superior to placebo for improving BCVA in people with chronic CSCR after 12 months of treatment. Ophthalmologists who currently prescribe eplerenone for CSCR should discontinue this practice.
FUNDING
Efficacy and Mechanism Evaluation Programme, and National Institute for Health Research and Social Care.
中文翻译:

依普利酮治疗活动性、先前未治疗疾病超过 4 个月 (VICI) 的慢性中心性浆液性脉络膜视网膜病变:一项随机、双盲、安慰剂对照试验。
背景在慢性中心性浆液性脉络膜视网膜病变 (CSCR) 中,液体在视网膜下腔积聚。CSCR 是一种常见的视力障碍疾病,可在 60 岁以下的个体中发展,并且没有明确的治疗方法。先前的研究表明盐皮质激素受体拮抗剂依普利酮对治疗 CSCR 有效;但是,该药物未获准用于治疗 CSCR 患者。我们旨在评估依普利酮在改善慢性 CSCR 患者视力方面是否优于安慰剂。方法 这项随机、双盲、平行组、多中心安慰剂对照试验在英国的 22 家医院进行。如果参与者年龄在 18-60 岁之间,并且接受过 4 个月或更长时间的初治 CSCR,则他们符合条件。患者被随机分配(1:1) 由试验统计学家通过密码保护的在线系统向依普利农或安慰剂组发送。分配按最佳矫正视力 (BCVA) 和医院进行分层。给予患者口服依普利酮(25 毫克/天,持续 1 周,增加至 50 毫克/天,持续长达 12 个月)加常规护理或安慰剂加常规护理长达 12 个月。所有参与者、护理团队、结果评估员、药剂师和试验管理组成员都对治疗分配不知情。主要结果是 12 个月时的 BCVA,以字母阅读来衡量。除安全性外的所有结果均在改进的意向治疗基础上进行分析(在主要分析人群和大多数次要分析人群中排除了在未提供随机化后 BCVA 测量的情况下撤回同意的参与者)。该试验已在 ISRCTN 注册,ISRCTN92746680,并已完成。结果 在 2017 年 1 月 11 日至 2018 年 2 月 22 日期间,我们招募并随机分配了 114 名患者接受依普利酮 (n=57) 或安慰剂 (n=57)。安慰剂组的三名参与者在没有提供随机化后 BCVA 测量值的情况下撤回同意,并被排除在主要结果分析人群之外。依普利农组的所有患者和安慰剂组的 54 名患者均被纳入主要结局。12 个月时的建模平均 BCVA 在安慰剂组为 79·5 个字母(SD 4·5),在依普利农组为 80·4 个字母(4·6),调整后的估计平均差异为 1·73 个字母(95% CI -1·12 至 4·57;p=0·24) 在 12 个月时。每组有 8 名 (14%) 患者出现高钾血症。依普利农组未报告严重不良事件,安慰剂组报告了三起不相关的严重不良事件(心肌梗塞[预期]、憩室炎[未预期]和代谢手术[未预期])。解释 在治疗 12 个月后,依普利农在改善慢性 CSCR 患者的 BCVA 方面并不优于安慰剂。目前为 CSCR 开具依普利酮的眼科医生应停止这种做法。资金有效性和机制评估计划,
更新日期:2020-01-24
中文翻译:

依普利酮治疗活动性、先前未治疗疾病超过 4 个月 (VICI) 的慢性中心性浆液性脉络膜视网膜病变:一项随机、双盲、安慰剂对照试验。
背景在慢性中心性浆液性脉络膜视网膜病变 (CSCR) 中,液体在视网膜下腔积聚。CSCR 是一种常见的视力障碍疾病,可在 60 岁以下的个体中发展,并且没有明确的治疗方法。先前的研究表明盐皮质激素受体拮抗剂依普利酮对治疗 CSCR 有效;但是,该药物未获准用于治疗 CSCR 患者。我们旨在评估依普利酮在改善慢性 CSCR 患者视力方面是否优于安慰剂。方法 这项随机、双盲、平行组、多中心安慰剂对照试验在英国的 22 家医院进行。如果参与者年龄在 18-60 岁之间,并且接受过 4 个月或更长时间的初治 CSCR,则他们符合条件。患者被随机分配(1:1) 由试验统计学家通过密码保护的在线系统向依普利农或安慰剂组发送。分配按最佳矫正视力 (BCVA) 和医院进行分层。给予患者口服依普利酮(25 毫克/天,持续 1 周,增加至 50 毫克/天,持续长达 12 个月)加常规护理或安慰剂加常规护理长达 12 个月。所有参与者、护理团队、结果评估员、药剂师和试验管理组成员都对治疗分配不知情。主要结果是 12 个月时的 BCVA,以字母阅读来衡量。除安全性外的所有结果均在改进的意向治疗基础上进行分析(在主要分析人群和大多数次要分析人群中排除了在未提供随机化后 BCVA 测量的情况下撤回同意的参与者)。该试验已在 ISRCTN 注册,ISRCTN92746680,并已完成。结果 在 2017 年 1 月 11 日至 2018 年 2 月 22 日期间,我们招募并随机分配了 114 名患者接受依普利酮 (n=57) 或安慰剂 (n=57)。安慰剂组的三名参与者在没有提供随机化后 BCVA 测量值的情况下撤回同意,并被排除在主要结果分析人群之外。依普利农组的所有患者和安慰剂组的 54 名患者均被纳入主要结局。12 个月时的建模平均 BCVA 在安慰剂组为 79·5 个字母(SD 4·5),在依普利农组为 80·4 个字母(4·6),调整后的估计平均差异为 1·73 个字母(95% CI -1·12 至 4·57;p=0·24) 在 12 个月时。每组有 8 名 (14%) 患者出现高钾血症。依普利农组未报告严重不良事件,安慰剂组报告了三起不相关的严重不良事件(心肌梗塞[预期]、憩室炎[未预期]和代谢手术[未预期])。解释 在治疗 12 个月后,依普利农在改善慢性 CSCR 患者的 BCVA 方面并不优于安慰剂。目前为 CSCR 开具依普利酮的眼科医生应停止这种做法。资金有效性和机制评估计划,











































 京公网安备 11010802027423号
京公网安备 11010802027423号