当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility profile of imatinib in pure and mixed solvents and calculation of thermodynamic propertires
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jct.2019.106031 Zehui Yang , Danfeng Shao , Guoquan Zhou
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jct.2019.106031 Zehui Yang , Danfeng Shao , Guoquan Zhou
|
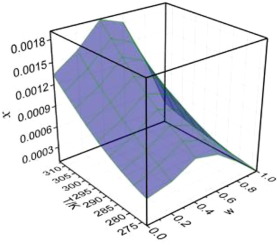
Abstract The pharmaceutical salt of imatinib prepared with low purity did not meet the pharmaceutically acceptable quality. The objective of this work is to research the shape of the solubility profile of imatinib in pure and mixed solvents at T = 273.15–313.15 K. At a given temperatures (from 273.15 K to 313.15 K), the largest the solubility is in toluene (2.205 × 10−3 at 313.15 K) and the lowest is in acetonitrile (9.739 × 10−5 at 313.15 K), it decrease based on the following order in different solvents: toluene > n-propanol > acetone > ethyl acetate > acetonitrile. In system of (acetone + n-propanol), the solubility of imatinib gave an increase trend until w = 0.2 firstly, and then decreased with the increasing mass fraction of acetone. Regarding for the system of (ethyl acetate + n-propanol), it reached to the maximum (1.902 × 10−3 at 313.15 K) at w = 0.4. Two thermodynamic models including modified Apelblat equation and Jouyban-Acree model were used to correlate. The maximum values of RAD calculated by modified Apelblat equation are all less than 1.66 × 10−2, and it is no more than 2.28 × 10−2 observed in Jouyban-Acree model for mixed solvents. Meanwhile, the positive values of apparent dissolution enthalpy ( Δ H sol o ) and apparent dissolution entropy ( Δ S sol o ) indicate that the dissolution process is not only endothermic but also entropy-driving.
中文翻译:

伊马替尼在纯溶剂和混合溶剂中的溶解度曲线和热力学特性的计算
摘要 低纯度制备的伊马替尼药用盐不符合药学上可接受的质量。这项工作的目的是研究伊马替尼在 T = 273.15–313.15 K 时在纯溶剂和混合溶剂中的溶解度曲线形状。在给定的温度下(从 273.15 K 到 313.15 K),在甲苯中的溶解度最大( 2.205 × 10−3 at 313.15 K),最低的是乙腈(9.739 × 10−5 at 313.15 K),在不同溶剂中按以下顺序降低:甲苯>正丙醇>丙酮>乙酸乙酯>乙腈。在(丙酮+正丙醇)体系中,伊马替尼的溶解度呈上升趋势,直至w=0.2,然后随着丙酮质量分数的增加而下降。对于(乙酸乙酯+正丙醇)体系,达到最大值(1. 902 × 10−3 at 313.15 K) at w = 0.4。使用两个热力学模型,包括修正的 Apelblat 方程和 Jouyban-Acree 模型进行关联。修正Apelblat方程计算的RAD最大值均小于1.66×10-2,在Jouyban-Acree模型中观察到的混合溶剂不超过2.28×10-2。同时,表观溶解焓( Δ H sol o )和表观溶解熵( Δ S sol o )的正值表明溶解过程不仅是吸热的,而且是熵驱动的。28 × 10−2 在 Jouyban-Acree 模型中观察到的混合溶剂。同时,表观溶解焓( Δ H sol o )和表观溶解熵( Δ S sol o )的正值表明溶解过程不仅是吸热的,而且是熵驱动的。28 × 10−2 在 Jouyban-Acree 模型中观察到的混合溶剂。同时,表观溶解焓( Δ H sol o )和表观溶解熵( Δ S sol o )的正值表明溶解过程不仅是吸热的,而且是熵驱动的。
更新日期:2020-05-01
中文翻译:

伊马替尼在纯溶剂和混合溶剂中的溶解度曲线和热力学特性的计算
摘要 低纯度制备的伊马替尼药用盐不符合药学上可接受的质量。这项工作的目的是研究伊马替尼在 T = 273.15–313.15 K 时在纯溶剂和混合溶剂中的溶解度曲线形状。在给定的温度下(从 273.15 K 到 313.15 K),在甲苯中的溶解度最大( 2.205 × 10−3 at 313.15 K),最低的是乙腈(9.739 × 10−5 at 313.15 K),在不同溶剂中按以下顺序降低:甲苯>正丙醇>丙酮>乙酸乙酯>乙腈。在(丙酮+正丙醇)体系中,伊马替尼的溶解度呈上升趋势,直至w=0.2,然后随着丙酮质量分数的增加而下降。对于(乙酸乙酯+正丙醇)体系,达到最大值(1. 902 × 10−3 at 313.15 K) at w = 0.4。使用两个热力学模型,包括修正的 Apelblat 方程和 Jouyban-Acree 模型进行关联。修正Apelblat方程计算的RAD最大值均小于1.66×10-2,在Jouyban-Acree模型中观察到的混合溶剂不超过2.28×10-2。同时,表观溶解焓( Δ H sol o )和表观溶解熵( Δ S sol o )的正值表明溶解过程不仅是吸热的,而且是熵驱动的。28 × 10−2 在 Jouyban-Acree 模型中观察到的混合溶剂。同时,表观溶解焓( Δ H sol o )和表观溶解熵( Δ S sol o )的正值表明溶解过程不仅是吸热的,而且是熵驱动的。28 × 10−2 在 Jouyban-Acree 模型中观察到的混合溶剂。同时,表观溶解焓( Δ H sol o )和表观溶解熵( Δ S sol o )的正值表明溶解过程不仅是吸热的,而且是熵驱动的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号