当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, synthesis and evaluation of new 4-arylthiazole-2-amine derivatives as acetylcholinesterase inhibitors.
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2020-01-24 , DOI: 10.1016/j.bmcl.2020.126985 Yuan Xu 1 , Meng-Meng Jian 1 , Chuang Han 1 , Kan Yang 1 , Li-Gai Bai 1 , Fei Cao 1 , Zheng-Yue Ma 1
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2020-01-24 , DOI: 10.1016/j.bmcl.2020.126985 Yuan Xu 1 , Meng-Meng Jian 1 , Chuang Han 1 , Kan Yang 1 , Li-Gai Bai 1 , Fei Cao 1 , Zheng-Yue Ma 1
Affiliation
|
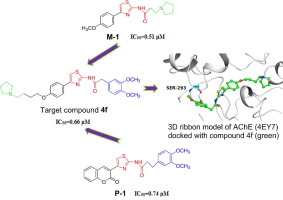
A series of new 4-arylthiazole-2-amine derivatives as acetylcholinesterase inhibitors (AChEIs) were designed and synthesized, Furthermore, their inhibitory activities against acetylcholinesterase in vitro were tested by Ellman spectrophotometry, and the results of inhibitory activity test showed that most of them had a certain acetylcholinesterase inhibitory activity in vitro. Moreover, the IC50 value of compound 4f was to 0.66 μM, which was higher than that of Rivastigmine and Huperzine-A as reference compounds, and it had a weak inhibitory effect on butyrylcholinesterase. The potential binding mode of compound 4f with AChE was investigated by the molecular docking, and the results showed that 4f was strongly bound up with AChE with the optimal conformation, in addition, their binding energy reached -11.27 Kcal*mol-1. At last, in silico molecular property of the synthesized compounds were predicted by using Molinspiration online servers. It can be concluded that the lead AChEIs compound 4f presented satisfactory drug-like characteristics.
中文翻译:

设计,合成和评估作为乙酰胆碱酯酶抑制剂的新型4-芳基噻唑-2-胺衍生物。
设计并合成了一系列新型的4-芳基噻唑-2-胺衍生物作为乙酰胆碱酯酶抑制剂(AChEIs),并通过Ellman分光光度法测试了它们对乙酰胆碱酯酶的体外抑制活性,抑制活性测试结果表明,它们中的大多数在体外具有一定的乙酰胆碱酯酶抑制活性。另外,化合物4f的IC 50值为0.66μM,高于作为参考化合物的Rivastigmine和石杉碱-A的IC 50值,并且对丁酰胆碱酯酶的抑制作用较弱。通过分子对接研究了化合物4f与AChE的潜在结合方式,结果表明4f以最佳构象与AChE牢固结合,另外结合能达到-11.27 Kcal * mol-1。最后,使用Molinspiration在线服务器预测了合成化合物的硅分子性质。可以得出结论,铅AChEIs化合物4f表现出令人满意的类药物特性。
更新日期:2020-01-24
中文翻译:

设计,合成和评估作为乙酰胆碱酯酶抑制剂的新型4-芳基噻唑-2-胺衍生物。
设计并合成了一系列新型的4-芳基噻唑-2-胺衍生物作为乙酰胆碱酯酶抑制剂(AChEIs),并通过Ellman分光光度法测试了它们对乙酰胆碱酯酶的体外抑制活性,抑制活性测试结果表明,它们中的大多数在体外具有一定的乙酰胆碱酯酶抑制活性。另外,化合物4f的IC 50值为0.66μM,高于作为参考化合物的Rivastigmine和石杉碱-A的IC 50值,并且对丁酰胆碱酯酶的抑制作用较弱。通过分子对接研究了化合物4f与AChE的潜在结合方式,结果表明4f以最佳构象与AChE牢固结合,另外结合能达到-11.27 Kcal * mol-1。最后,使用Molinspiration在线服务器预测了合成化合物的硅分子性质。可以得出结论,铅AChEIs化合物4f表现出令人满意的类药物特性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号