当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of ex situ chronopotentiometric analysis on stability of bovine serum albumin on mercury electrodes
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.jelechem.2020.113884 Veronika Ostatná , Ryan M. West
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.jelechem.2020.113884 Veronika Ostatná , Ryan M. West
|
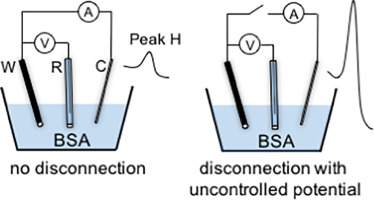
Abstract Constant current chronopotentiometric stripping (CPS) allows analysis of proteins based on measured peak H resulting from catalytic hydrogen evolution reaction. The technique is label-free and sensitive to the structure and stability of the protein adsorbed at the mercury electrode. Comparison of proteins must be carried out under the same conditions, including ionic strength, temperature, pH, and accumulation potential and time, as all of these factors can affect the protein stability and structure on the electrode surface. Here we show that for bovine serum albumin, uncontrolled disconnection of the cell after accumulation, as is necessary for ex situ CPS measurements, can cause an increased susceptibility to electric field-induced denaturation during the subsequent CPS measurement. This destabilization is attributed to oxidation of the Hg electrode during disconnection. For this reason, care much be taken when ex situ CPS measurement of protein is performed.
中文翻译:

异位计时电位分析对牛血清白蛋白在汞电极上稳定性的影响
摘要 恒流计时电位剥离 (CPS) 允许基于催化析氢反应产生的测量峰 H 分析蛋白质。该技术是无标记的,并且对吸附在汞电极上的蛋白质的结构和稳定性敏感。蛋白质的比较必须在相同的条件下进行,包括离子强度、温度、pH、积累电位和时间,因为所有这些因素都会影响电极表面的蛋白质稳定性和结构。在这里,我们表明,对于牛血清白蛋白,非原位 CPS 测量所必需的积累后细胞不受控制的断开会导致在随后的 CPS 测量期间对电场诱导的变性的敏感性增加。这种不稳定归因于断开连接期间汞电极的氧化。因此,在进行蛋白质的异位 CPS 测量时要格外小心。
更新日期:2020-03-01
中文翻译:

异位计时电位分析对牛血清白蛋白在汞电极上稳定性的影响
摘要 恒流计时电位剥离 (CPS) 允许基于催化析氢反应产生的测量峰 H 分析蛋白质。该技术是无标记的,并且对吸附在汞电极上的蛋白质的结构和稳定性敏感。蛋白质的比较必须在相同的条件下进行,包括离子强度、温度、pH、积累电位和时间,因为所有这些因素都会影响电极表面的蛋白质稳定性和结构。在这里,我们表明,对于牛血清白蛋白,非原位 CPS 测量所必需的积累后细胞不受控制的断开会导致在随后的 CPS 测量期间对电场诱导的变性的敏感性增加。这种不稳定归因于断开连接期间汞电极的氧化。因此,在进行蛋白质的异位 CPS 测量时要格外小心。











































 京公网安备 11010802027423号
京公网安备 11010802027423号